Show results for
Refine by
Bowel Equipment Supplied In Usa Massachusetts
10 equipment items found
Manufactured by:Finch Therapeutics Group, Inc. based inSomerville, MASSACHUSETTS (USA)
In collaboration with Takeda Pharmaceuticals, we are developing FIN-524 and FIN-525, orally administered, Targeted Consortia product candidates designed for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD), respectively. UC and CD are the two most common types of IBD, an autoimmune condition that causes inflammation of the gastrointestinal (GI) ...
Manufactured by:Vedanta Biosciences, Inc based inCambridge, MASSACHUSETTS (USA)
VE202 is an orally administered rationally-defined bacterial consortium candidate being developed for the potential treatment of inflammatory bowel disease (IBD). VE202 consists of clonal human commensal bacteria strains selected for their ability to impact the number and activity of regulatory T cells in the gut mucosa and manufactured under cGMP ...
Manufactured by:Peters Surgical based inBoulogne-Billancourt, FRANCE
Mini invasive abdominal wall perforation (no additional trocar incision). Reduced degree of Trendelenburg position for the patient (eg bowels ...
Manufactured by:Ardigen based inKraków, POLAND
This process can be applied to various scoring systems, such as the Nancy and Geboes Histological score (for inflammatory bowel disease data) or the Gleason score (for prostate cancer ...
Manufactured by:Peters Surgical based inBoulogne-Billancourt, FRANCE
Mini invasive abdominal wall perforation (no additional trocar incision). Reduced degree of Trendelenburg position for the patient (eg bowels retraction). . ...
by:Iterative Health, Inc. based inCambridge, MASSACHUSETTS (USA)
Save time and energy with AI Documentation. Our AI-enhanced documentation technology may soon make it easier to develop more detailed and accurate procedure documentation—all without increasing the burden on ...
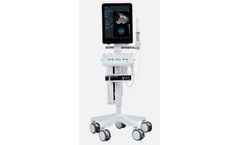
Manufactured by:BK Medical Holding Company, Inc. based inBurlington, MASSACHUSETTS (USA)
Fusing powerful real-time ultrasound images with pre-exam MRI data gives you better guidance to help accurately target lesions detected on the MRI images, potentially reducing the risk of missing high-grade tumors or under-staging tumors for active ...
by:TISSIUM based inParis, FRANCE
Our proprietary biomorphic programmable polymers enable tissue reconstruction and can be adapted for use in multiple clinical ...
Manufactured by:Sofregen Medical Inc based inFramingham, MASSACHUSETTS (USA)
SERI® Surgical Scaffold was cleared for use in the U.S. in April of 2013. SERI® provides predictable tissue regeneration, transferring load-bearing responsibility within 12 months. SERI® Surgical Scaffold is indicated for use as a transitory scaffold for soft tissue support and repair to reinforce deficiencies where weakness or voids exist that require the addition of material to ...
Manufactured by:Emulate based inBoston, MASSACHUSETTS (USA)
Investigate mechanisms of colon inflammation and barrier disruption and evaluate the efficacy of anti-inflammatory drug candidates in an organoid-derived ...