- Home
- Equipment
- usa massachusetts
- clinical candidate developed
Show results for
Refine by
Applications
Clinical Candidate Developed Equipment Supplied In Usa Massachusetts
17 equipment items found
Manufactured by:Medicem Group a.s. based inPraha 10, CZECH REPUBLIC
In this area, MEDICEM leverages its patented material platform featuring a unique hydrogel designed to expand under pressure. DILAPAN-S is a synthetic osmotic hygroscopic dilator for the uterine cervix made from proprietary anisotropic xerogel. The embodiment is a sterile synthetic gel rod, which absorbs fluid from the tissue of the cervical canal, resulting in reversible dehydration and ...
by:Verb Biotics based inBoston, MASSACHUSETTS (USA)
Yso1 is a clinical stage development program aiming at harnessing the potential of Christensenella minuta as a groundbreaking biotherapy to treating obesity and metabolic disorders. The goal of our disruptive approach is to return to obese patients christensenella, which have the unique ability to repair the unbalanced microbiome, thereby restoring impaired key ...
by:Vor Biopharma based inCambridge, MASSACHUSETTS (USA)
Licensed from the National Institutes of Health, VCAR33 is a CD33-directed chimeric antigen receptor T cell (CAR-T) therapy. A T cell therapy using the same CAR construct as VCAR33 is being studied in a multi-site Phase 1/2 clinical trial as an autologous monotherapy bridge-to-transplant for relapsed and/or refractory AML patients, sponsored by the National Marrow Donor Program ...
by:Vertex Pharmaceuticals based inBoston, MASSACHUSETTS (USA)
Vertex is focused on developing transformative medicines for people with serious and life-threatening diseases. To do this, we conduct clinical trials to assess the safety and effectiveness of investigational medicines, which, if established, will help us obtain approvals from regulatory authorities. These approvals, in turn, are required before medicines can be made widely available to ...
Manufactured by:MorphoSys AG based inPlanegg, GERMANY
Pelabresib (CPI-0610) is an investigational selective small-molecule designed to promote anti-tumor activity by inhibiting the function of bromodomain and extra-terminal domain (BET) proteins to decrease the expression of abnormally expressed genes in cancer. The compound has demonstrated a wide therapeutic window, with activity seen at a 48 mg dose in a lymphoma study and with a maximum ...
by:Kephera Diagnostics, LLC based inFramingham, MASSACHUSETTS (USA)
At Kephera, we are developing rapid tests for infectious diseases using novel immunochemistry in point-of-care format. Our proprietary assay technology is based on principles of particle interaction combined with a simple device, which together enable rapid detection of specific antibodies or antigens associated with the target pathogen. The broadly enabling technology is applicable to a wide ...
Manufactured by:Finch Therapeutics Group, Inc. based inSomerville, MASSACHUSETTS (USA)
CP101, an orally administered, Complete Consortia product candidate in late-stage clinical development for the prevention of recurrent C. difficile infection is also being developed for the treatment of chronic HBV. Chronic HBV infection occurs when the initial immune response fails to clear the virus, and may result in ...
Manufactured by:Ariana Pharma based inParis, FRANCE
Ariana® has consolidated multiple, publicly available administrative healthcare admissions databases into a locally searchable observational health data resource of patient hospitalization, outpatient and other episodes, comprising in excess of 6B records. ...
by:Intellia Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
The first patient was dosed with our investigational genome editing treatment for transthyretin (ATTR) amyloidosis in November 2020. Our modular approach enables us to optimize the power and versatility of the CRISPR/Cas9 technology and, importantly, allows us to rapidly develop therapeutics for numerous diseases that currently have limited treatment ...
by:Valo Health, Inc. based inBoston, MASSACHUSETTS (USA)
Opal is the first end-to-end integrated drug discovery & development platform. Traditional, linear approaches to target discovery and drug development have created sub-optimized systems that are intrinsically disintegrated and ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-155 is an investigational, oral, rationally designed, fermented microbiome therapeutic designed to reduce the incidence of gastrointestinal infections, bacteremia, and graft versus host disease (GvHD) in immunocompromised patients, including patients receiving allogeneic hematopoietic stem cell transplantation ...
Manufactured by:MorphoSys AG based inPlanegg, GERMANY
Tafasitamab (MOR208) is a humanized FC-modified CD19 targeting immunotherapy in clinical development for the treatment of B cell malignancies. CD19 is broadly expressed on the surface of B cells. It is therefore considered as a potential target for the treatment of B cell malignancies, such as non-Hodgkin’s lymphoma (NHL), including diffuse large B cell lymphoma (DLBCL), indolent lymphomas ...
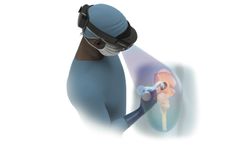
by:HipXpert based inBoston, MASSACHUSETTS (USA)
The HipInsight system represents a significant advancement in the field of orthopedic surgery, specifically for total hip arthroplasty. As the first mixed reality-based digital surgery platform cleared by the FDA for this purpose, the system provides surgeons with a unique capability often likened to 'x-ray vision.' This innovative technology allows medical professionals to visualize and navigate ...
Manufactured by:Transgene based inIllkirch-Graffenstaden, FRANCE
TG4001 is an innovative therapy capable of combating papillomavirus-induced cancers. The concept behind TG4001 is to teach the immune system to recognise and destroy the cancer cells expressing HPV-16 antigens, specifically E6 and E7 ...
Manufactured by:Biotest AG based inDreieich, GERMANY
At the ISTH 2020 Biotest presented its novel recombinant Factor VIII molecules overcoming the major challenges of current Factor VIII replacement therapy by combining a significantly extended half-life, low immunogenicity and the possibility of either subcutaneous or intravenous ...
Manufactured by:Biotest AG based inDreieich, GERMANY
Trimodulin (BT588, predecessor BT086) is a human plasma-derived native polyvalent antibody preparation for intravenous administration. Trimodulin contains immunoglobulins IgM (-23%), IgA (~ 21%) and igG (-56%). Trimodulin mediates its mode of action via three potential mechanisms which are; opsonization of causal pathogens, neutralizing of microbial pathogens and their virulence factors (endo- ...
Manufactured by:Biotest AG based inDreieich, GERMANY
Trimodulin is an innovative polyvalent antibody composition, purified from human plasma. In comparison to standard IgG preparations (IVIG), trimodulin contains relevant amounts of IgM and IgA in addition to ...