- Home
- Equipment
- usa massachusetts
- clinical phase
Show results for
Refine by
Clinical Phase Equipment Supplied In Usa Massachusetts
19 equipment items found
by:Verb Biotics based inBoston, MASSACHUSETTS (USA)
Using a revolutionary form of living biotherapy targeting the lung, YSOPIA is pioneering the innovative field of the lung microbiome. As part of this program, YSOPIA has selected a clinical candidate having demonstrated strong in vivo efficacy on inflammation and lung functions. We have achieved the formulation feasibility and cleared the regulatory hurdles associated with ...
Manufactured by:Vedanta Biosciences, Inc based inCambridge, MASSACHUSETTS (USA)
VE303 is an orally administered, rationally-defined bacterial consortium candidate being developed for high-risk Clostridioides difficile (CDI) infection. VE303 consists of 8 types of clonal human commensal bacteria strains selected for their ability to provide colonization resistance to C. difficile and manufactured under cGMP conditions. It is produced under cGMP conditions from pure, clonal ...
Manufactured by:AOBiome Therapeutic, LLC based inCambridge, MASSACHUSETTS (USA)
We have completed six Phase 2 clinical programs and multiple pre-clinical programs. Over 1,000 patients have been enrolled in our clinical trials since 2016. All of those patients except for 28 in our open label pediatric study have been enrolled in randomized double-blind placebo controlled clinical trials ...
Manufactured by:Acorda Therapeutics, Inc. based inPearl River, NEW YORK (USA)
GGF2 is a member of the neuregulin growth factor family, and has been shown to promote recovery after neurological injury, as well as to enhance heart function in animal models of heart failure. GGF2 showed positive effects on cardiac function in the two Phase 1 clinical studies conducted to date. The Phase 1b trial assessed three ...
by:Cartesian Therapeutics based inGaithersburg, MASSACHUSETTS (USA)
Building upon the proven success of SEL-212, ImmTOR has exciting potential to give rise to antigen-specific immunity across a range of immunogenic compounds when co-administered with biologic therapies like ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-109 is a first-in-class investigational microbiome therapeutic administered orally following antibiotics to reduce recurrence of C. difficile infection (CDI) in patients with recurrent ...
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
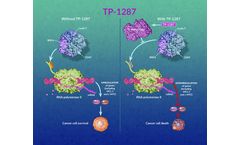
TP-1287 is an investigational oral CDK9 inhibitor that has shown favorable oral bioavailability in preclinical models. TP-1287 is enzymatically cleaved, yielding the active moiety, a potent inhibitor of CDK9.35 Inhibiting CDK9 is thought to downregulate the transcription of target genes, including MCL-1, reducing leukemic blast viability in MCL-1–dependent hematologic malignancies, and ...
Manufactured by:MorphoSys AG based inPlanegg, GERMANY
Constellation Pharmaceuticals, a MorphoSys company, is using a starting dose of 125 mg in MANIFEST, our global, multicenter, open-label Phase 2 study of pelabresib in patients with Myelofibrosis (MF). Preclinical studies and translational insights from our first-in-human study of pelabresib led us to prioritize the clinical development of pelabresib in ...
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
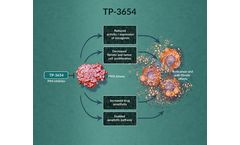
TP-3654 is an oral investigational inhibitor of PIM kinases, which has potential antitumor and anti-fibrotic effects through multiple pathways, including induction of ...
by:Myeloid Therapeutics based inCambridge, MASSACHUSETTS (USA)
Emerging from the Company’s proprietary ATAK™ CAR receptor library, Myeloid’s novel in vivo engineering platform specifically targets and activates myeloid cells to elicit broader anti-tumor adaptive immunity. Through this approach, Myeloid demonstrates that delivery of lipid-nanoparticles (LNPs) encapsulating mRNA results in uptake and selective expression by myeloid cells in ...
Manufactured by:Alphageneron based inCambridge, MASSACHUSETTS (USA)
An autologous mHsp70 targeted NK Cell Therapy that activates NK cells to target and kill cells expressing mHsp70. The therapy begins with selecting patients whom have high mHsp70 expression with our Companion Diagnostic AP-CDx (described below), collecting their peripheral blood mononuclear cells (PBMCs) by leukapheresis, ex vivo activation of PBMCs with our synthetic Hsp70-derived peptide ...
Manufactured by:Kalaris based inWaltham, MASSACHUSETTS (USA)
Kalaris Therapeutics is focused on developing innovative treatments for retinal diseases, aiming to reduce the burden of frequent clinical visits for patients with retinal neovascular and exudative conditions. Their lead product, TH103, is a fully humanized, recombinant fusion protein designed for intravitreal delivery as an anti-VEGF agent. TH103 functions as a soluble decoy ...
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
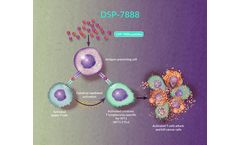
Ombipepimut-S Emulsion (DSP-7888) is an investigational immunotherapeutic cancer vaccine containing 2 peptides that induce WT1-specific cytotoxic T lymphocytes (WT1-CTLs) and helper T cells to attack WT1-expressing cancerous cells found in various types of hematologic malignancies and solid tumors.1,2 Researchers have identified that adding helper T-cell–inducing peptides improved ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-301 is an investigational, oral, rationally-designed, fermented microbiome therapeutic for the treatment of mild-to-moderate ulcerative colitis (UC). SER-301 is a consortium of multiple bacterial strains that is manufactured by fermenting each strain individually and then combining to form drug product. ...
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
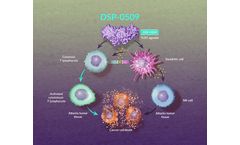
DSP-0509 is an investigational Toll-like receptor (TLR) 7 agonist, which is hypothesized to induce cytokine production, activate cytotoxic T lymphocytes, and sustain immune-mediated antitumor ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-287 is a consortium of multiple bacterial spores manufactured by fractionating and purifying targeted bacteria from stool of healthy human donors. SER-287 has been granted Fast Track designation and Orphan Drug designation by the FDA. A completed SER-287 Phase 1b clinical study demonstrated a statistically significant difference in the ...
by:Intellia Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
The first patient was dosed with our investigational genome editing treatment for transthyretin (ATTR) amyloidosis in November 2020. Our modular approach enables us to optimize the power and versatility of the CRISPR/Cas9 technology and, importantly, allows us to rapidly develop therapeutics for numerous diseases that currently have limited treatment ...
Manufactured by:Invicro, LLC based inNeedham, MASSACHUSETTS (USA)
Invicro has spent 20 years specializing in neuroscience imaging solutions for preclinical, early phase and late phase clinical studies. Our capabilities span from in vitro assays to in vivo imaging using PET, SPECT and MRI imaging biomarkers in CNS drug development and CNS clinical trials. We have expertise in coordinating ...
Manufactured by:Alphageneron based inCambridge, MASSACHUSETTS (USA)
An allogenic NK cell therapy that targets tumors expressing mHsp70. Patients are selected with high mHsp70 exosomes in their blood using our Companion Diagnostic AP-CDx (described below). We obtain PBMCs from healthy donors by apheresis, their NK cells are expanded, and incubated ex vivo with TKD and IL-2, followed by freezing, thawing and intravenous administration to ...