- Home
- Equipment
- usa massachusetts
- harvesting
Refine by
Harvesting Equipment Supplied In Usa Massachusetts
7 equipment items found
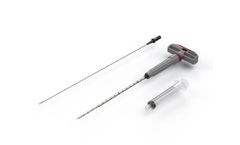
Manufactured by:Cervos Medical based inMarshfield, MASSACHUSETTS (USA)
The length makes it ergonomically friendly for accessing the iliac crest and can be advanced wither manually or with a mallet. This device is often used in conjunction with the CER-BN-84 bone dowel harvesting device and CF-G10 bone graft granules. Combining marrow aspirate with autogenous bone and a graft extender allows clinicians to fill boney defects using the patient’s ...
Manufactured by:Cervos Medical based inMarshfield, MASSACHUSETTS (USA)
The T-Handle allows it to be advanced either manually or with a mallet. This device is often used in conjunction with the CER-BN-116 bone dowel harvesting device and CF-G5 bone graft granules. Combining marrow aspirate with autogenous bone and a graft extender allows clinicians to fill boney defects using the patient’s own cells as the engine for bone healing. The ...
Manufactured by:Cervos Medical based inMarshfield, MASSACHUSETTS (USA)
Its shorter length makes it ergonomically friendly for working in extremity locations and it incorporates patented technology that limits peripheral blood dilution and eliminates the need for centrifugation. This device is often used in conjunction with the CER-BN-116 bone dowel harvesting device and CF-G5 bone graft granules. Combining marrow aspirate with autogenous bone and ...
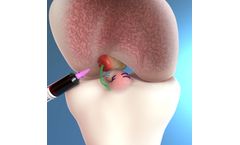
Manufactured by:Miach Orthopaedics, Inc. based inWestborough, MASSACHUSETTS (USA)
The BEAR Implant is an ideal bridge designed to restore the native ACL. Validated by Level 1 clinical evidence, No harvest site morbidity or need for donor tissue, Easy, reproducible procedure, Indicated for a broad range of ACL tear types. The BEAR Implant is a decellularized, bovine-derived, type I collagen implant that resorbs within 8 weeks of implantation. ...
Manufactured by:MorphoSys AG based inPlanegg, GERMANY
Peptides are an increasingly important class of drug. As natural ligands to many targets, they can combine agonistic or antagonistic activity with low toxicity risk and can be applied to disease targets, where small molecules or antibody-based drugs cannot be used. However, the number of therapeutic peptide drugs is held back by properties that are inherent to wild-type peptides. Namely, that ...
Manufactured by:Finch Therapeutics Group, Inc. based inSomerville, MASSACHUSETTS (USA)
CP101, an orally administered, Complete Consortia product candidate in late-stage clinical development for the prevention of recurrent C. difficile infection is also being developed for the treatment of chronic HBV. Chronic HBV infection occurs when the initial immune response fails to clear the virus, and may result in inflammation of hepatocytes, and complications including cirrhosis, liver ...
Manufactured by:Finch Therapeutics Group, Inc. based inSomerville, MASSACHUSETTS (USA)
Our lead product candidate, CP101, is an orally administered, Complete Consortia product candidate initially targeting the prevention of recurrent CDI. The Centers for Disease Control and Prevention considers CDI to be one of the top three most urgent antibiotic resistant threats and the most common cause of healthcare associated infection in the United ...