- Home
- Equipment
- usa massachusetts
- nasopharyngeal swab
Refine by
Nasopharyngeal Swab Equipment Supplied In Usa Massachusetts
6 equipment items found
Manufactured by:Rhinostics Inc. based inBoston, MASSACHUSETTS (USA)
Rhinostics GrooveSwab brings novel materials to nasopharyngeal swab collection, providing more comfortable collection. The polypropylene, proprietary honeycomb collection head completely collects viral sample, allows for dry transport, and rapid and complete elution of viral particles from the nasopharyngeal ...
Manufactured by:Rhinostics Inc. based inBoston, MASSACHUSETTS (USA)
Rhinostics GrooveSwab brings novel materials to nasopharyngeal swab collection, providing more comfortable collection. The polypropylene, proprietary honeycomb collection head completely collects viral sample, allows for dry transport, and rapid and complete elution of viral particles from the nasopharyngeal ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
cIntended Use: Fosun COVID-19 RT-PCR Detection Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in upper and lower respiratory specimens (such as anterior nasal swabs, mid-turbinate nasal swabs, nasopharyngeal swabs, oropharyngeal swabs, sputum, lower ...
Manufactured by:LaunchWorks CDMO based inBeverly, MASSACHUSETTS (USA)
LaunchWorks VTM with CDC recipe is now available with storage at 8 – 30° C for 24 months. In stock. Available for immediate delivery. Made in Beverly, ...
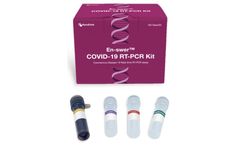
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
The En-swer™ COVID-19 RT-PCR Kit detects E, N, and RNaseP genes of SARS-CoV-2 qualitatively through real-time reverse-transcription polymerase chain reaction. It can be used for rapid detection and outbreak control of COVID-19. ...
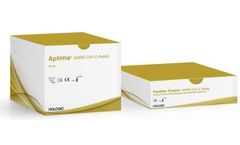
Manufactured by:Hologic, Inc. based inMarlborough, MASSACHUSETTS (USA)
The emergence of SARS-CoV-2 was unforeseen, and the worldwide outbreak of COVID-19 has already impacted millions. In response to the desperate public need for rapid high-volume testing we developed SARS-CoV-2 assays on both our Panther® and Panther Fusion® systems.* Both assays enable labs to run over 1000 tests in 24 hours and attain first results in 3.5 hours or ...