- Home
- Equipment
- usa massachusetts
- neuropathy
Refine by
Neuropathy Equipment Supplied In Usa Massachusetts
5 equipment items found
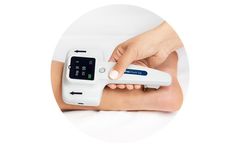
Manufactured by:NeuroMetrix, Inc. based inWoburn, MASSACHUSETTS (USA)
An accurate and fast point-of-care test for detecting a common form of nerve degeneration called peripheral neuropathy. DPNCheck is a fast, accurate, and quantitative nerve conduction test that is used to evaluate nerves for evidence of peripheral neuropathy, such as diabetic peripheral neuropathy (DPN). It is designed to be used by clinicians at ...
Manufactured by:Exergen Corporation based inWatertown, MASSACHUSETTS (USA)
The DermaTemp is especially recommended for use in plastic and vascular surgery, pain management, rheumatology, neurology, anesthesiology, oncology, and of particular interest in wound management, especially as used in diabetic ...
by:Intellia Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
The first patient was dosed with our investigational genome editing treatment for transthyretin (ATTR) amyloidosis in November 2020. Our modular approach enables us to optimize the power and versatility of the CRISPR/Cas9 technology and, importantly, allows us to rapidly develop therapeutics for numerous diseases that currently have limited treatment ...
Manufactured by:Mersana Therapeutics based inCambridge, MASSACHUSETTS (USA)
UpRi, a first-in-class ADC targeting the sodium-dependent phosphate transport protein NaPi2b, utilizes the Dolaflexin platform to deliver about 10 DolaLock payload molecules per antibody. The NaPi2b antigen is broadly expressed in ovarian cancer and non-small cell lung cancer (NSCLC) adenocarcinoma. UpRi is being studied in UPLIFT, a single-arm registration strategy, in patients with ...
Manufactured by:Eagle Pharmaceuticals, Inc. based inWoodcliff Lake, NEW JERSEY (USA)
Indication: PEMFEXY is indicated in combination with cisplatin for the initial treatment of patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC). PEMFEXY is indicated as a single agent for the maintenance treatment of patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) whose disease has not progressed after four ...