- Home
- Equipment
- usa massachusetts
- therapeutic crawling device
Show results for
Refine by
Therapeutic Crawling Device Equipment Supplied In Usa Massachusetts
12 equipment items found
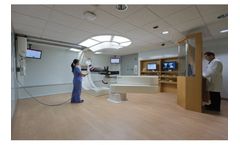
Manufactured by:Mevion Medical Systems based inLittleton, MASSACHUSETTS (USA)
The MEVION S250™ provides next-generation proton therapy. Built on the world’s only gantry-mounted proton accelerator, the MEVION S250 delivers a highly stable, uniform proton beam that is simple to deliver. The MEVION S250 has been treating patients since 2013 at multiple treatment sites across the ...
Manufactured by:Mevion Medical Systems based inLittleton, MASSACHUSETTS (USA)
The MEVION S250mx™ is a scalable proton therapy solution with two, three or four room options. Because of Mevion’s unique core technology, each treatment room is fully independent. This gives you the flexibility to add rooms as patient volume grows, allowing you to adapt to increasing demand, minimize financial risk, and maximize your clinical and operational ...
Manufactured by:Signifier Medical Technologies Ltd based inLondon, UNITED KINGDOM
eXciteOSA® is the world’s first daytime therapy proven to strengthen weak tongue muscles known to be the root cause of sleep apnea and ...
Manufactured by:Neutron Therapeutics, Inc. based inDanvers, MASSACHUSETTS (USA)
Neutron Therapeutics has developed an accelerator-based, in-hospital neutron source to replace the previously required nuclear reactor. This source is composed of a 2.6 MeV electrostatic proton accelerator and a rotating, solid lithium target for generating neutrons. Neutron Therapeutics will provide this neutron source as part of a comprehensive therapeutic treatment suite, that will combine all ...
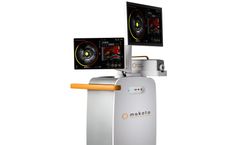
Manufactured by:Infraredx, Inc. based inBedford, MASSACHUSETTS (USA)
There are still broad swaths of uncharted territories concerning the role lipid core plaques play in heart disease. Now, you can achieve unparalleled insights with the Dualpro™ IVUS+NIRS catheter and its accompanying Makoto Intravascular Imaging System, the only FDA-cleared dual-modality catheter and imaging system indicated for the detection of lipid core plaque (LCP) and the ...
Manufactured by:NxStage Medical, Inc. based inLawrence, MASSACHUSETTS (USA)
System One S offers higher dialysate flow rates designed to provide a wider range of therapy options to meet all of your patients' unique clinical needs. Whether your patients are working professionals, students, or retired, System One S offers flexible therapy solutions to fit each of their unique lifestyles. System One and System One S let patients play an active role in their healthcare by ...
Manufactured by:Hill-Rom Holdings, Inc. based inChicago, ILLINOIS (USA)
The Volara System can make it faster and easier to deliver OLE therapy to a broader range of patients throughout the hospital—and even at ...
Manufactured by:Mevion Medical Systems based inLittleton, MASSACHUSETTS (USA)
The MEVION S250i™ with HYPERSCAN™ pencil beam scanning technology delivers high-quality, robust Intensity Modulated Proton Therapy (IMPT) treatments at hyper-speed and without ...
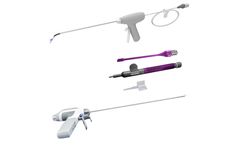
Manufactured by:Viant Medical based inFoxborough, MASSACHUSETTS (USA)
Energy-sealing devices, dissection devices, and RF therapy devices for procedural areas including: Gastrointestinal, Urologic, Prostate care, Women’s health, Phaco needles and lens ...
Manufactured by:Mersana Therapeutics based inCambridge, MASSACHUSETTS (USA)
UpRi, a first-in-class ADC targeting the sodium-dependent phosphate transport protein NaPi2b, utilizes the Dolaflexin platform to deliver about 10 DolaLock payload molecules per antibody. The NaPi2b antigen is broadly expressed in ovarian cancer and non-small cell lung cancer (NSCLC) adenocarcinoma. UpRi is being studied in UPLIFT, a single-arm registration strategy, in patients with ...
Manufactured by:AVEO Pharmaceuticals, Inc. based inBoston, MASSACHUSETTS (USA)
FOTIVDA (tivozanib) is an oral, once-daily, differentiated vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI). It is a potent, selective inhibitor of VEGFRs 1, 2, and 3 with a long half-life designed to improve efficacy and tolerability.1,2 AVEO received U.S. Food and Drug Administration (FDA) approval for FOTIVDA in March 2021 for the treatment of adult patients ...
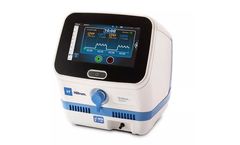
Manufactured by:Belmont Medical Technologies based inBillerica, MASSACHUSETTS (USA)
How quickly you start cooling a patient impacts survival and neurological function, especially after a hypoxic-ischemic encephalopathy (HIE) diagnosis or cardiac arrest. That’s why clinicians call on CritiCool, a simple and effective thermal regulating ...