- Home
- Equipment
- usa michigan
- implanted
Refine by
Implanted Equipment Supplied In Usa Michigan
35 equipment items found
Manufactured by:Shoulder Innovations based inGrand Rapids, MICHIGAN (USA)
The InSet Glenoid Implant was designed to address the number one issue in Total Shoulder Arthroplasty, Glenoid ...
Manufactured by:Shoulder Innovations based inGrand Rapids, MICHIGAN (USA)
The InSet™ Glenoid Implant was designed to address the number one issue in Total Shoulder Arthroplasty, Glenoid ...
Manufactured by:Shoulder Innovations based inGrand Rapids, MICHIGAN (USA)
The InSet PLUS augmented glenoid offers surgeons the ability to dial the augment to the area of greatest defect. The InSet PLUS was designed to offer additional correction of the glenoid as it allows for the combination of eccentric reaming with an additional 5 & 10 degrees of correction. Like the standard InSet Glenoid, the InSet PLUS Augmented Glenoid has the same flat back design, which ...
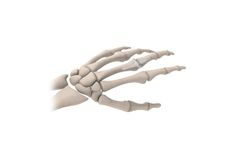
Manufactured by:BioPro, Inc based inPort Huron, MICHIGAN (USA)
The Osteotec Silicone Finger Implant is a single-piece flexible joint replacement implant for the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints in the hand; commonly due to rheumatoid or ...
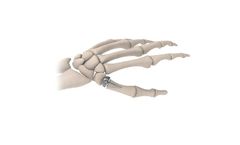
Manufactured by:BioPro, Inc based inPort Huron, MICHIGAN (USA)
The BioPro Modular Thumb Implant is a two-piece implant consisting of a head and a press-fit plasma-sprayed stem. The Modular Thumb is designed to address carpometacarpal (CMC) joint arthritis and the challenges with past implants including dislocation, implant loosening, and ...
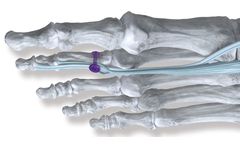
Manufactured by:Precision Edge Surgical Products based inSault Sainte Marie, MICHIGAN (USA)
Go ahead. Challenge us with an incredibly demanding thread form. Not only can we produce solid and cannulated implantable screws and bone taps in diameters ranging from 1-6 mm in lengths up to 16 inches, but we use product-simulation software to show you pre-production review of thread forms. It’s all part of the process that requires us to consider quality above all. We ...
Manufactured by:Stryker based inKalamazoo, MICHIGAN (USA)
OrthoSensor, Inc. is proud to have partnered with leading joint replacement companies Smith & Nephew, Stryker and Zimmer Biomet, allowing VERASENSE to be utilized in more replacement procedures around the world. VERASENSE is compatible for use with the following knee systems: Smith & Nephew Legion® and Journey II®, Stryker® Triathlon®, and Zimmer Biomet Persona®, ...
Premium
Manufactured by:AMS, Inc. based inAmerican Falls, IDAHO (USA)
AMS Vapor Implants have double woven stainless steel wire screens with a .0057-in. (0.15mm ...
Premium
Manufactured by:AMS, Inc. based inAmerican Falls, IDAHO (USA)
AMS 6-inches (152mm) Gas Vapor Implant Installation Kit are used for permanent soil gas monitoring, UST monitoring, groundwater sampling, air sparging, vapor extraction monitoring, and as a pressure measurement point in vacuum testing. This kit allows a user to obtain or collect a soil gas sample by positioning or placing a 6” implant in the subsurface, and ...
Manufactured by:Paragon 28, Inc. based inEnglewood, COLORADO (USA)
TenoTac® is a patent-pending procedure and soft tissue fixation system designed to permanently address the source of the toe contracture. Cannulated instrumentation allows for accurate and reproducible placement of the implant. A threaded titanium implant with a spiked base is used to achieve and hold soft tissue fixation. The procedure requires minimal steps ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Non-bone tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. Non-bone tendons used in ACL reconstruction procedures allow for multiple fixation ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's bone-tendon-bone (BTB) grafts are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These grafts allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Matrix HD Allograft Dermis is an acellular human dermis allograft sterilized to a Sterility Assurance Level (SAL) of 10-6 using the Tutoplast Tissue Sterilization Process. This proprietary process retains the three-dimensional intertwined multidirectional fibers and mechanical properties of the native dermis tissue. The Matrix HD graft provides a natural scaffold to support the body's ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's Achilles tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These tendons allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Conventional allografts are used for various procedures to fill bony voids or gaps in a patient's skeletal system, restore segmental bone loss due to trauma, osteoporosis, removal of tumors or to aid in the surgical correction for a deformity. These grafts provide a scaffold for bone ingrowth to allow for remodeling with the patient’s own bone. These grafts are pre-cut to select sizes to ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical offers the only meniscal allograft with both bone and meniscus sterilized through the BioCleanse® Tissue Sterilization Process. The BioCleanse processed meniscus graft provides a sterilized alternative to traditional aseptically processed meniscus allografts and sets the standard for safety and ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical’s fresh-stored osteochondral (OC) allografts enable surgeons to resurface osteochondral defects with mature hyaline cartilage and repair subchondral bone in a single procedure. Fresh grafts are cleansed, processed and preserved to maintain chondrocyte ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Fortiva Porcine Dermis is a non-crosslinked acellular porcine dermal matrix offering a safe and natural biologic option. Fortiva implants are processed through the Tutoplast® Tissue Sterilization Process and terminally sterilized via validated low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. Fortiva Porcine Dermis is a ready-to-use biologic ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Cortiva Allograft Dermis is a non-crosslinked acellular dermal matrix offering a safe and natural biologic option and sterilized to a sterility assurance level (SAL) of 10-6 through the Tutoplast® Tissue Sterilization ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their ...