- Home
- Equipment
- usa minnesota
- clinical studies
Show results for
Refine by
Clinical Studies Equipment Supplied In Usa Minnesota
17 equipment items found
Manufactured by:INOVIQ Ltd based inNotting Hill, AUSTRALIA
INOVIQ has the exclusive worldwide license for SubB2M - an engineered protein that binds to a unique sugar molecule called Neu5Gc which is only present in human cancers. In initial clinical studies, SubB2M has detected cancers with 100% sensitivity and specificity for mid to late-stage cancers, and >95% specificity and 100% sensitivity for early-stage cancers. ...
Manufactured by:Tactile Medical based inMinneapolis, MINNESOTA (USA)
A comfortable, convenient way to effectively manage lymphedema. The Flexitouch arm-shoulder garment is designed to treat patients with lymphedema in the comfort of their own ...
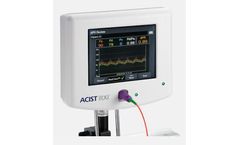
Manufactured by:ACIST Medical Systems based inEden Prairie, MINNESOTA (USA)
Non-hyperemic index for coronary physiology. ACIST diastolic pressure ratio (dPR), using the ACIST RXi Rapid Exchange System and Navvus MicroCatheter, provides a non-hyperemic alternative for physiological assessment of coronary ...
Manufactured by:Rebiotix Inc., a Ferring Company based inRoseville, MINNESOTA (USA)
Rebiotix Inc., a Ferring Company, has leveraged its clinical, manufacturing and regulatory experience with RBX2660 to develop the first of its kind non-frozen, room temperature stable oral microbiota-based formulation under the MRT™ drug platform, known as RBX7455. While it is currently used to reduce the incidence of recurrent Clostridioides difficile infection, additional indications are ...
Manufactured by:Tactile Medical based inMinneapolis, MINNESOTA (USA)
The leading at-home therapy device for the treatment of lymphedema. The Flexitouch system is used by more than 100,000 patients to self-manage lymphedema and nonhealing venous leg ulcers. Flexitouch Plus is clinically proven to decrease the chronic swelling associated with ...
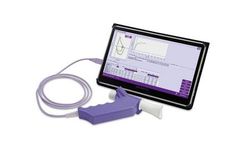
Manufactured by:Biomedix based inSaint Paul, MINNESOTA (USA)
This device is designed to be plugged into a PC, offering a fully-integrated experience from the computer. It includes enhanced provocation and challenge testing to streamline the coaching needed to produce consistent results. A disposable, one-use spirette is used to prevent contamination to the ...
Manufactured by:Rebiotix Inc., a Ferring Company based inRoseville, MINNESOTA (USA)
Rebiotix Inc., a Ferring Company, has conducted several trials of the lead microbiota-based product in the MRT drug platform, RBX2660, for reducing the rate of recurrence of Clostridioides difficile infection. RBX2660 is currently under study in a Phase 3 clinical trial in conjunction with the US Food and Drug Administration’s Investigational New Drug ...
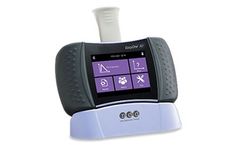
Manufactured by:Biomedix based inSaint Paul, MINNESOTA (USA)
This portable device can be used when connected to a PC or laptop, or can be used in a standalone capacity, and data can be synced with a PC, then synced with the Biomedix Xchange population health management platform. A disposable, one-use FlowTube is used to prevent contamination to the ...
Manufactured by:MGC Diagnostics Corporation based inSaint Paul, MINNESOTA (USA)
Individuals who cannot perform typical spirometry tests, such as the elderly, pediatric or patients with neuromuscular disease are often the patients who need accurate test results the most. That's where the Resmon PRO FULL (V3) comes in. With just normal breathing from the patient, the Resmon PRO FULL (V3) can measure the mechanical properties of the respiratory system and provide a simple, ...
Manufactured by:Rebiotix Inc., a Ferring Company based inRoseville, MINNESOTA (USA)
There is also increasing evidence that the gut microbiota plays an important role in protecting the host from disease. Clinical and scientific studies indicate that disturbances to the gut microbiota, often referred to as “dysbiosis,” may be involved in infectious, inflammatory, metabolic, liver and neurological diseases. Until recently, antibiotics ...
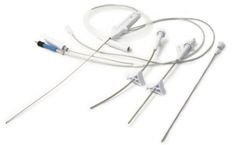
Manufactured by:Clarus Medical, LLC based in55441, MINNESOTA (USA)
Small 1.7mm catheter: Integrates illumination, imaging, irrigation, outflow and therapeutic Ho:YAG laser fiber. Full visibility: Physician can identify target tissue, observe tissue effects and verify the amount of tissue removed. Truly minimally invasive: Little or no skin scar with low morbidity. Continuous irrigation: Keeps the disc cool and provides copious flushing to remove disc material. ...
Manufactured by:Carbon Medical Technologies, Inc. based inSt. Paul, MINNESOTA (USA)
Durasphere is an injectable bulking agent containing pyrolytic carbon-coated graphite beads suspended in a water-based carrier gel containing beta glucan. It is used in the treatment of stress urinary incontinence. Durasphere is a highly biocompatible, permanent material that can be administered in less than 30 minutes in a simple office-based ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
TYVASO (treprostinil) is a prostacyclin mimetic indicated for the treatment of: Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%). The ...
Manufactured by:Sanuwave and Sanuwave Health, Inc. based inSuwanee, GEORGIA (US) (USA)
SANUWAVE’s orthoPACE System uses PACE Technology, and has a long and successful history of treating bone conditions requiring osteogenesis, calcific joints, conditions causing painful joints, as well as treating chronic pain caused by musculoskeletal disorders and inflammatory conditions. This system is designed for specialists in orthopedics, trauma, and sport injuries ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Unituxin is a GD2-binding monoclonal antibody indicated, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and 13-cis-retinoic acid (RA), for the treatment of pediatric patients with high-risk neuroblastoma who achieve at least a partial response to prior first-line multiagent, multimodality ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Remodulin is a prostacyclin vasodilator indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts ...
Manufactured by:MGC Diagnostics Corporation based inSaint Paul, MINNESOTA (USA)
The HypAir offers a wide range of pulmonary function tests in a single system. With high quality open circuit spirometry, lung volumes, diffusion, respiratory mechanics and more, the HypAir is the ideal device for complete, modular pulmonary function ...