- Home
- Equipment
- usa minnesota
- implanted
Show results for
Refine by
Implanted Equipment Supplied In Usa Minnesota
45 equipment items found
Manufactured by:Medtimo Inc based inEden Prairie, MINNESOTA (USA)
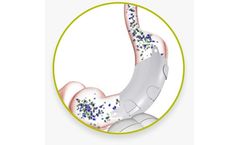
The ReShape Vest™ is a revolutionary, anatomy-friendly, laparoscopic, implantable device to enable weight loss and stomach preservation. The procedure restricts stomach volume without cutting, stapling, or removing the ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
The OptiMesh implant expands in three dimensions when filled to create an anatomy-conforming interbody fusion implant. By containing and hyper-compacting the graft, OptiMesh uses granular mechanics to transform the graft into a large load-bearing pack capable of restoring anatomy, providing indirect decompression, resisting subsidence, and promoting ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
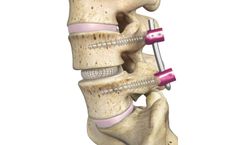
The Threshold Pedicular Fixation System offers a robust, yet simple, method of delivering anatomy-conserving pedicular fixation. The system features provide:Secure implant-to-instrumentation connections. The screw extensions grip the inside and outside surfaces of the screw head. This encapsulation ensures the extension cannot splay off of the screw head as the rod is reduced ...
Manufactured by:Anteris Technologies Limited based inToowong, AUSTRALIA
Evidence of Long-Term Durability: 25 patients were followed for up to 10 years; No graft failure, thromboembolic events, infections or device-related reinterventions; Echocardiographic assessment demonstrated the absence of calcification in all ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
The Threshold V2 Pedicular Fixation System was designed to efficiently treat a wide variety of spinal pathologies through an open or guide wireless MIS approach. The system features a multi-start, quick advancing corticocancellous screw thread pattern, a wide variety of implant options, and intuitively designed instrumentation. ...
Manufactured by:Cirtec Medical based inBrooklyn Park, MINNESOTA (USA)
Setting the Pace for CRM: Cardiac rhythm management devices continue to evolve rapidly in form factor, reliability and lifespan. Even as implantable designs become increasingly compact and functional, they are introducing groundbreaking new elements like leadless pacing and integrated fixation concepts for minimally invasive deployment. Cardiac rhythm management devices such as ...
Manufactured by:Reprise Biomedical based inPlymouth, MINNESOTA (USA)
Our proprietary perfusion decellularization process involves cannulating the main vasculature of the organ and perfusing a mild detergent solution through the native blood vessels to result in a preserved native scaffold containing the appropriate strength and microenvironment for cellular integration after it is implanted in the patient’s ...
Manufactured by:Nextern Inc based inWhite Bear Lake, MINNESOTA (USA)
We’ve worked with partners on steerable balloon catheters, steerable neurovascular devices, and delivery devices for structural heart implants. As your vertically-integrated partner, we have the end-to-end services to guide you through the entire product development ...
Manufactured by:Carbon Medical Technologies, Inc. based inSt. Paul, MINNESOTA (USA)
Bone markers serve as reference points for an imaging system to increase the ease and accuracy of a surgical procedure. Bone-implanted markers allow for increased imaging contrast and are unable to move, providing greater precision throughout the treatment ...
Manufactured by:Cirtec Medical based inBrooklyn Park, MINNESOTA (USA)
Vascotube, a Cirtec company, based in Birkenfeld, Germany, processes Nitinol tubing used in implantable medical devices including: Heart Valves, TAVR and TMVR Stents, Peripheral Vascular Stents, Neurovascular ...
Manufactured by:TissX based inMinneapolis, MINNESOTA (USA)
The development of percutaneous cardiac valves (aortic, mitral, tricuspid and pulmonary). The development of surgically implanted cardiac valves. Pericardial Patches and other tissue for surgical, vascular and other applications. Functional components for structural heart disease treatment and other vascular uses. ...
Manufactured by:Biomerics based inSalt Lake City, UTAH (USA)
We’ve worked with customers on steerable balloon catheters, steerable neurovascular devices, and delivery devices for structural heart implants, to name a few. As your vertically integrated partner, we have the end-to-end services to guide you through the entire product development ...
Manufactured by:Vascudyne, Inc. based inStillwater, MINNESOTA (USA)
TRUE Tissue developed from cells isolated from donor tissue and is 100% biological. There are no synthetic materials or chemical fixation used, and implanted tissues are completely cell-derived and acellular. That’s how we make tissues that are TRUE to ...
Manufactured by:Carbon Medical Technologies, Inc. based inSt. Paul, MINNESOTA (USA)
This marker is manufactured with a slightly denser core material allowing for enhanced daily images. This marker can be especially helpful on larger patients or patients with bony or implant interference with treatment ...
Manufactured by:Carbon Medical Technologies, Inc. based inSt. Paul, MINNESOTA (USA)
BiomarC, our legacy marker, is an implantable fiducial marker. It provides an extremely valuable method to ensure accurate and repeatable target positioning in radiation therapy with little perturbation of the radiation beam. This exclusive marker is manufactured with our least dense nonmetallic material. ...
Manufactured by:Reprise Biomedical based inPlymouth, MINNESOTA (USA)
MiroFlex (formerly known as MiroMesh) is a non-crosslinked acellular surgical matrix, derived from the highly vascularized porcine liver. It is intended for implantation to reinforce soft tissue where weakness exists in patients requiring soft tissue repair such as hernia repairs or reinforcement in plastic and reconstructive surgery. If larger sizes of MiroFlex are desired, ...
Manufactured by:Formacoat based inChaska, MINNESOTA (USA)
Stents, Films up to 17” (41 cm) wide, Micro-Catheters, Drug Delivery Catheters, Stent Delivery-System Catheters, Embolic Capture Devices, Medical Device Balloons, Component Level Materials, Hypo Tubes, Micro-well plates, IOL Insertion Cartridges and other ophthalmic devices. And, in some situations, medical device ...
by:Minnesota MedTec based inMinneapolis, MINNESOTA (USA)
Balloon catheters are manufactured in our certified class 10,000 controlled environments. Most drawings and processes are already established for Nylon and PU balloons. Some designs are made using adhesive bonded components and some of them are designed to use heat bonds based on the products performance ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
The Elite™ Expandable Interbody Fusion System represents the next generation of expandable technology. Its unique design minimizes both neural retraction and insertion force, accommodates a larger graft channel than many competitive expandable cages, and provides controlled expansion to restore disc height, potentially providing indirect decompression. When paired with Incite Cortical ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
Minimally invasive, bone-sparing surgical methods and large load-bearing interbody fusion systems have dramatically reduced the biomechanical demands placed on fixation systems, eliminating the need for large complex spinal fixation constructs for many patients. The Capture Facet Fixation System is the perfect complement. ...