- Home
- Equipment
- usa minnesota
- neurology
Show results for
Refine by
Neurology Equipment Supplied In Usa Minnesota
19 equipment items found
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
Stealth Autoguide cranial robotic guidance platform is engineered to provide stereotactic positioning and trajectory guidance. Stealth Autoguide™ cranial robotic guidance platform accurately aligns to your surgical plans for cranial ...
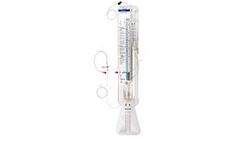
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
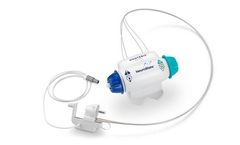
The Duet External Drainage and Monitoring System is a CSF drainage system that can be used for both external ventricular and lumbar ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
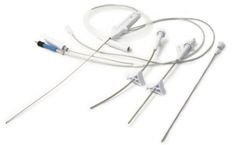
The React™ 68 catheter and the React™ 71 catheter feature a coil and braid design along with end-to-end nitinol construction — easing navigation to the M1 and M2 segments. Combined with the Riptide™ aspiration system, these catheters are designed to revascularize patients experiencing acute ischemic ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Marathon™ flow directed microcatheter was designed for the infusion of therapeutic agents such as embolization materials and contrast media in tortuous, distal vessels.1 . The ApolloTM OnyxTM delivery microcatheter was expressly designed to access the neurovasculature for the controlled selective infusion of the OnyxTM liquid embolic system ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
Durepair Regeneration Matrix is a non-synthetic dura substitute used to repair defects in the ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The INVISx Cranial Fixation System is a unique, two-sided clamping cranial fixation device used for skull flap replacement following a craniotomy ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The first fully-assembled, disposable external ventricle drainage system on the market. The Becker External Drainage and Monitoring System is used to drain and monitor CSF flow from the patient’s lateral ventricles or the lumbar subarachnoid space to reduce intracranial pressure. The system can be used preoperatively, intraoperatively and postoperatively to monitor CSF chemistry, cytology ...
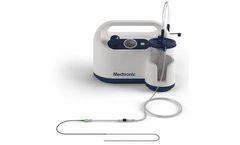
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Riptide Aspiration System is designed to retrieve a blood clot and restore blood flow to occluded vessels in the brain for patients experiencing acute ischemic stroke (AIS) due to a large vessel occlusion ...
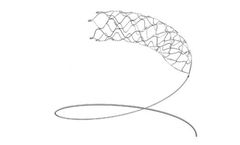
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Solitaire X revascularization device, featuring Parametric™ design, a unique overlapping stent retriever-based technology, restores blood flow and retrieves clots from occluded blood vessels in the brain for patients experiencing acute ischemic stroke (AIS) due to a large vessel occlusion ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
Designed for presurgical embolization of brain arteriovenous malformations (bAVMs), Onyx™ liquid embolic system (LES) is a pre-mixed, radiopaque, injectable embolic fluid consisting of the following components: EVOH (ethylene vinyl-alcohol copolymer), DMSO (dimethyl-sulfoxide) and TA (micronized tantalum ...
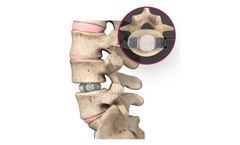
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
The system minimizes the amount and duration of retraction on the neural structures and surrounding soft tissues, both of which have been linked to neurologic deficits following lateral interbody fusion surgery.1, ...
Manufactured by:Rebiotix Inc., a Ferring Company based inRoseville, MINNESOTA (USA)
Clinical and scientific studies indicate that disturbances to the gut microbiota, often referred to as “dysbiosis,” may be involved in infectious, inflammatory, metabolic, liver and neurological diseases. Until recently, antibiotics were a common way to fight different diseases – now, the opportunity to restore gut microbes may provide an effective and durable ...
Manufactured by:Teleflex Medical OEM based inPlymouth, MINNESOTA (USA)
Teleflex Medical OEM specializes in ultra-small tubing solutions for peripheral vascular, cardiology, and neurology applications. They manufacture micro-diameter tubing with internal diameters ranging from 0.002” to 0.091” and wall thickness from 0.003” to 0.007”. Their product portfolio includes reinforced and conductor-embedded tubing designs made from ...
Manufactured by:WR Medical Electronics Co. based inMaplewood, MINNESOTA (USA)
The CASE IV QST lab focuses on the testing of sensory abnormalities in the areas of temperature change sensation, vibration, and pain threshold testing. ...
Manufactured by:Monteris Medical based inMinnetonka, MINNESOTA (USA)
The Robotic Probe Driver, designed for use with a skull anchor like the Monteris Mini-Bolt, reduces profile in the MRI for access to virtually any brain location along the ideal ...
Manufactured by:Monteris Medical based inMinnetonka, MINNESOTA (USA)
Dependable skull fixation for neurosurgical devices. The only cranial fixation bolt with a robotic interface to enable precise laser delivery. ...
Manufactured by:Teleflex Medical OEM based inPlymouth, MINNESOTA (USA)
At Teleflex Medical OEM, insulated fine wire is a specialty. Our team is ready to create a customized solution for your application. We have a proprietary, polymer coating process that uses a variety of insulation and wire materials. Wire can be coated with a single, heavy, triple, or quad build. Our advanced technologies allow us to manufacture wire smaller than a human ...
Manufactured by:Clarus Medical, LLC based in55441, MINNESOTA (USA)
Small 1.7mm catheter: Integrates illumination, imaging, irrigation, outflow and therapeutic Ho:YAG laser fiber. Full visibility: Physician can identify target tissue, observe tissue effects and verify the amount of tissue removed. Truly minimally invasive: Little or no skin scar with low morbidity. Continuous irrigation: Keeps the disc cool and provides copious flushing to remove disc material. ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Unituxin is a GD2-binding monoclonal antibody indicated, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and 13-cis-retinoic acid (RA), for the treatment of pediatric patients with high-risk neuroblastoma who achieve at least a partial response to prior first-line multiagent, multimodality ...