- Home
- Equipment
- usa new york
- administration of drug
Refine by
Administration Of Drug Equipment Supplied In Usa New York
26 equipment items found
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Dalbavancin (INN, trade name Dalvance) is a novel second-generation lipoglycopeptide antibiotic. It belongs to the same class as vancomycin.The Food and Drug Administration approved dalbavancin for the treatment of acute bacterial skin and skin structure infections caused by certain susceptible ...
by:Stemline Therapeutics, Inc. based inNew York, NEW YORK (USA)
ELZONRISTM (tagraxofusp, SL-401) — ELZONRIS is a novel targeted therapy directed to the interleukin-3 (IL-3) receptor-α (CD123), a target present on a wide range of malignancies. ELZONRIS was approved by the US Food and Drug Administration (FDA) on December 21, 2018, for the treatment of adult and pediatric patients, 2 years and older, with blastic ...
Manufactured by:Zetagen Therapeutics Inc. based inSyracuse, NEW YORK (USA)
ZetaMet is a synthetic, small-molecule, inductive biologic technology being developed to target and resolve metastatic bone lesions while inhibiting future tumor growth and regenerating bone. The small molecule has been approved by the U.S. Food and Drug Administration (FDA) since 1971. Zetagen scientists have discovered an entirely new pathway for this ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
In October 2018, SIGA entered into a Cooperative Research and Development Agreement (CRADA) with the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) with a focus on Good Laboratory Practice (GLP) studies of TPOXX® for PEP using a U.S. Food and Drug Administration (FDA)-approved non-human primate model of TPOXX® efficacy ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
SIGA completed clinical trials for the IV formulation with funding from the Biomedical Advanced Research and Development Authority (BARDA). In April 2021, SIGA submitted a New Drug Application (NDA) with the U.S. Food and Drug Administration (FDA) for IV TPOXX®. It was formally approved by FDA in May 2022. ...
Manufactured by:Tonix Pharmaceuticals Holding Corp. based inChatham, NEW JERSEY (USA)
TNX-601 ER is a novel oral formulation of tianeptine hemioxalate that is being developed as a potential treatment for major depressive disorder (MDD), posttraumatic stress disorder (PTSD) and neurocognitive dysfunction associated with corticosteroid use. Tonix’s TNX-601 ER includes tianeptine hemioxalate in an extended-release ...
Manufactured by:Zetagen Therapeutics Inc. based inSyracuse, NEW YORK (USA)
ZetaFuse is the first synthetic, small molecule, osteo-inductive biologic, to safely aid bone healing of spinal fusions. The U.S. Food and Drug Administration (FDA) has awarded ZetaFuse Breakthrough Device Designation[1] for the complete spinal column. ZetaFuse is driven by the activation of a novel molecular pathway, by leveraging this patented mechanism, to ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
Oral TPOXX® (tecovirimat) is the first drug approved by the U.S. Food and Drug Administration (FDA) that is specifically indicated for the treatment of smallpox disease in adults and pediatric patients weighing at least 13 kg. (Prescription Label) TPOXX® inhibits viral maturation of variola virus (the virus that causes smallpox) and other ...
Manufactured by:Pfizer Inc. based in, NEW YORK (USA)
The Pfizer-BioNTech COVID-19 vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 16 years of age and older. The emergency use of this product is only authorized for ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed VesiculoVax™-vectored vaccines for pre- and post-exposure protection against the hemorrhagic disease caused by multiple species of filovirus (i.e. Zaire ebolavirus, Sudan ebolavirus and Marburg ...
by:Curemark based inRye Brook, NEW YORK (USA)
Centers for Disease Control and Prevention estimate that 1 in 68 children in the United States have been diagnosed with Autism. There is currently no approved drug on the market that treats the core symptoms of Autism. At Curemark, we are working to change that. Curemark’s lead drug candidate, CM-AT, has received “Fast Track” designation from ...
Manufactured by:Oramed Pharmaceuticals, Inc. based inNew York, NEW YORK (USA)
The attractive advantages of oral drug delivery, including increased patient comfort and compliance, reduced risk of infection, simpler application in pediatric medicine, first-pass metabolism preceding systemic exposure, and cost effectiveness, have positioned it the most popular and preferred route of drug ...
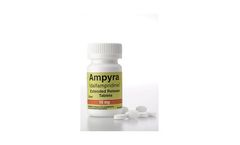
Manufactured by:Acorda Therapeutics, Inc. based inPearl River, NEW YORK (USA)
AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg is a prescription medicine approved by the U.S. Food and Drug Administration (FDA) to help improve walking in adults with multiple sclerosis MS. This was demonstrated by an increase in walking speed. ...
Manufactured by:Misonix based inFarmingdale, NEW YORK (USA)
Designed by nature, made for healing. Do you have a slow-healing or chronic wound? This could be a diabetic foot ulcer, a venous leg ulcer, or other difficult-to-heal wounds. It is important that these serious wounds with damaged skin receive the advanced care needed to avoid complications ranging from infection to even amputation. Application of TheraSkin — a real human skin allograft ...
Manufactured by:POP Biotechnologies, Inc. (POP BIO) based inBuffalo, NEW YORK (USA)
PhotoDOX is a revolutionary cancer therapy designed to address solid tumors which are resistant to current standards of care through the innovative benefits of the light-activated PoP-liposome drug delivery ...
by:Delcath Systems, Inc. based inNew York, NEW YORK (USA)
Delcath has developed a percutaneous targeted, whole-organ treatment that delivers a select high-dose chemotherapy agent directly to the liver, while controlling systemic side effects, by filtering the chemotherapeutic agent from the blood before returning it to the patient. In the US, our drug/device investigational product is HEPZATO™ KIT (melphalan hydrochloride for ...
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
This product contains Human Allograft Material (referred to as “Graft”) collected from human cadaveric donors. All tissue processed for Impladent Ltd. is recovered by U.S. tissue banks as well as a completed donor chart for the enclosed product including but not limited to: serology results, preprocessing culture results, medical and social history evaluation and serodilution ...
Manufactured by:Himed based inOld Bethpage, NEW YORK (USA)
MATRIX MCD® is a specialized biocompatible micro abrasive blasting solution designed for titanium and titanium alloy medical devices, particularly in the dental and orthopedic sectors. Utilizing a proprietary multi-phase calcium phosphate (MCD) abrasive, it delivers precise surface texturing without compromising the structural integrity of the implant. Unlike conventional abrasive blasting ...
Manufactured by:ErtelAlsop based inKingston, NEW YORK (USA)
The pharmaceutical and biotech markets require a high level of functional consistency from filtration products. To provide a higher level of confidence for these customers, ErtelAlsop drafted its Drug Master File and submitted it to the FDA Center for Drug Evaluation and Research. ErtelAlsop manufactures all filter pads to very high standards, and the Drug Master File provides the documentation ...
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
This product contains Human Allograft Material (referred to as “Graft”) collected from human cadaveric donors. All tissue processed for Impladent Ltd. is recovered by U.S. tissue banks as well as a completed donor chart for the enclosed product including but not limited to: serology results, preprocessing culture results, medical and social history evaluation and serodilution ...