- Home
- Equipment
- usa new york
- astm standard
Refine by
Astm Standard Equipment Supplied In Usa New York
10 equipment items found
Manufactured by:American Diagnostic Corporation (ADC) based inHauppauge, NEW YORK (USA)
Well balanced power handle that offers bright illumination. Satin Fiberoptic Handles feature: Light source in handle simplifies blade cleaning, allows for use of brighter, whiter light. Satin handles with a knurled finish for a positive grip. Color coded green to signify compatibility ONLY with fiberoptic blades. Available in traditional “C”, “AA”, and stubby ...
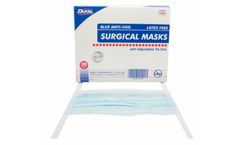
Manufactured by:Dukal Corporation based inRonkonkoma, NEW YORK (USA)
3-Ply pleated lightweight, breathable material. BFE of 99%. Meets ASTM Level 1 ...
Manufactured by:American Diagnostic Corporation (ADC) based inHauppauge, NEW YORK (USA)
The Adtemp Mini 432 is compact, lightweight, and fast. Offering an ingenious design and incredible portability – along with the safety and convenience of a non-contact instrument – the 432 is sure to become an essential instrument for busy professionals. ...
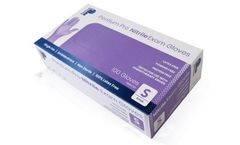
Manufactured by:NinoMed LLC based inSyracuse, NEW YORK (USA)
High quality, competitively priced Nitrile Examination Gloves that meet or Exceeds ASTM D6319. Our Pentium Pro™ gloves have also been tested for resistance to permeation of various chemotherapy drugs per ASTM D6978-05, “Standard Practice for Assessment of Resistance of Medical Gloves to Permeation by Chemotherapy ...
Manufactured by:Proactive Medical Products based inMount Vernon, NEW YORK (USA)
Proactive regulators feature a protective contents gauge and a built-in pressure relief valve for safety. 30 micron sintered bronze inlet filter for durability. Meets or exceeds accuracy standards for ASTM, American National, and CGA. Easy-to-operate single selector knob, laser etched flow orifices. Body material aluminum 6061-T6, anodized green. CGA 540 inlet ...
Manufactured by:Proactive Medical Products based inMount Vernon, NEW YORK (USA)
Proactive regulators feature a protective contents gauge and a built-in pressure relief valve for safety. 30 micron sintered bronze inlet filter for durability. Meets or exceeds accuracy standards for ASTM, American National, and CGA. Easy-to-operate single selector knob, laser etched flow orifices. Body material aluminum 6061-T6, anodized green. CGA 870 inlet ...
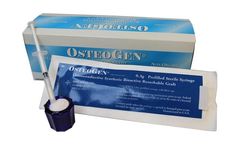
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
OsteoGen Synthetic Bioactive Resorbable crystals and crystal clusters in 300 to 400μ are osteoconductive, highly hydrophilic, resorbable, synthetically-derived non-ceramic form of hydroxylapatite, the major mineral component of bone and dental enamel. OsteoGen®, [Ca5(PO4)3(OH)] is used for the augmentation and repair in periodontal and oralmaxillofacial bone defects. ...
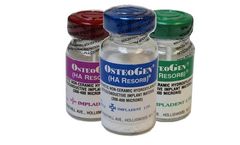
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
OsteoGen® Synthetic Bioactive Resorbable crystals and crystal clusters in 300 to 400μ are osteoconductive, highly hydrophilic, resorbable, synthetically-derived non-ceramic form of hydroxylapatite, the major mineral component of bone and dental enamel. OsteoGen®, [Ca5(PO4)3(OH)] is used for the augmentation and repair in periodontal and oralmaxillofacial bone ...
Manufactured by:Himed based inOld Bethpage, NEW YORK (USA)
Unlike natural forms, Himed's HA is synthetically manufactured to ensure consistent stoichiometry, essential for precise clinical and engineering applications. The company's HA is produced under GMP guidelines and conforms to ISO 13485:2016 and ASTM F1185 standards with a purity of ≥96%, ensuring reliable batch-to-batch consistency demanded by medical and ...
Manufactured by:Himed based inOld Bethpage, NEW YORK (USA)
Unlike conventional abrasive blasting methods, which may leave residual contaminants, MATRIX MCD® ensures a residue-free finish, complying with ASTM F86 passivation standards. This technology facilitates enhanced osseointegration by increasing bone-to-implant contact area, which is crucial for implant stability. It is applicable in both traditional ...