- Home
- Equipment
- usa new york
- bioptic
Refine by
Bioptic Equipment Supplied In Usa New York
10 equipment items found
Manufactured by:Electro Surgical Instrument Company (ESI) based inRochester, NEW YORK (USA)
Baby Tischler Biopsy Punch Forceps 8″ (20.3cm) shaft, 2 x 4mm bite with thumb ...
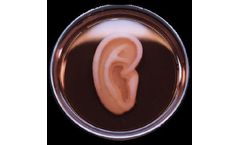
Manufactured by:3DBio Therapeutics based inLong Island City, NEW YORK (USA)
A small biopsy is performed on the patient’s microtia ear remnant and sent to 3DBio’s GMP manufacturing facility. The patient’s cartilage cells are isolated from the biopsy, expanded in cell culture, and then “printed” into the shape of the patient’s normal ear using 3DBio’s proprietary innovative printing technology. The components to accomplish this ...
Manufactured by:CDx Diagnostics Inc. based inSuffern, NEW YORK (USA)
AI-powered diagnostic platform to help prevent esophageal cancer. Included in the Standard of Practice Guidelines for the screening and surveillance of Barrett’s esophagus, WATS3D is our proprietary diagnostic platform for identifying precancerous cells in the ...
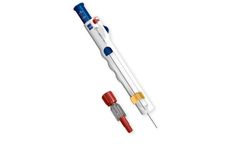
Manufactured by:AngioDynamics, Inc. based inLatham, NEW YORK (USA)
The BioSentry tract sealant system is the first biopsy sealant system of its kind designed specifically to address the issues of biopsy-related pneumothorax. The BioSentry tract sealant system deploys a self-expanding hydrogel plug into the pleural space following a percutaneous lung biopsy – creating an airtight seal that closes the pleural ...
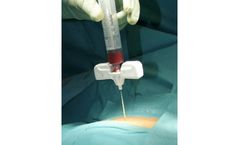
Manufactured by:Busse Hospital Disposables based inHauppauge, NEW YORK (USA)
Ultra-sharp and manufactured to the highest tolerances, the Busse Bone Marrow Biopsy Needle is designed to provide outstanding and consistent performance. With its substantial, easily gripped handle, our 11g x 4″ “J”-style needle provides the physician with greater torque than similar instruments. Combined with the ultra-sharp trocar tip on the stylet, Busse’s exclusive ...
Manufactured by:CDx Diagnostics Inc. based inSuffern, NEW YORK (USA)
A routine diagnostic test that empowers doctors to help prevent oral cancer and save lives. OralCDx allows you to painlessly test any white or red spot in the mouth to rule out the possibility that precancerous cells are present. If dysplasia is found, the cells can typically be removed in order to stop the progression of oral cancer. ...
Manufactured by:Rarecells Diagnostics SAS based inParis Cedex 06, FRANCE
Cancer is the second cause of death, accounting for nearly 9 million deaths in 2017 all over the world. In 2019, 1,762,450 new cancer cases and 606,880 cancer deaths are projected to occur in the United States. It is estimated that metastasis is the primary cause of 90% of all cancer-related deaths. Circulating Tumor Cells (CTCs) detection and analysis are the foundation of non-invasive tests ...
Manufactured by:Hemogenyx Pharmaceuticals plc based inLondon, UNITED KINGDOM
Hemogenyx Pharmaceuticals utilizes postnatal human hemogenic endothelial cells (Hu-PHEC cells), discovered by Hemogenyx Pharmaceuticals’ Co-Founder Dr Vladislav Sandler, to generate cancer-free hematopoietic stem cells (HSC) for use in transplants to treat blood cancers with superior results. A patent for Dr Sandler’s work was issued in the US in February ...
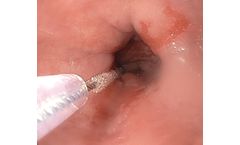
Manufactured by:CDx Diagnostics Inc. based inSuffern, NEW YORK (USA)
Provides physicians with a practical method of testing non-suspicious tissue in order to help prevent laryngeal cancer. Otolaryngologists are often faced with a dilemma when relatively non-suspicious tissue is observed in the office during flexible laryngoscopy. The probability that commonly observed tissue abnormalities are dysplastic is low, while a forceps biopsy to test these lesions presents ...
Manufactured by:miR Scientific, LLC based inNew York, NEW YORK (USA)
miR Scientific is a precision healthcare company committed to improving public health by transforming cancer management globally. The company’s proprietary miR Disease Management Platform® was developed to revolutionize the standard of value-based care for cancers and initially focuses on urological cancers. The initial test delivered by the miR Disease Management Platform® is the ...