- Home
- Equipment
- usa new york
- defibrillator
Refine by
Defibrillator Equipment Supplied In Usa New York
15 equipment items found
Manufactured by:Nissha Medical Technologies, Inc. based inBuffalo, NEW YORK (USA)
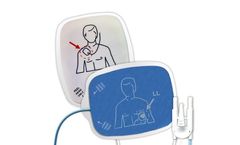
6600100H - 97 DEFIBRILLATOR PADS - 1 PER PK. Pad Size in Inches (L x W) : 4.7 x 6.1 in / 11.9 x 15.6 cm. Integrated Leadwires : 60 in / 1.5 M. Backing Material : Polyethylene Foam. Conductive Element Material : Tin Copper. Adapter Connector : Comparable to Physio OEM. ...
Manufactured by:Netech Corporation based inFarmingdale, NEW YORK (USA)
Delta 1600 AED tester is exclusively designed to test all automated External Defibrillators, including Pulsed AEDs (Schiller). ...
Manufactured by:Nissha Medical Technologies, Inc. based inBuffalo, NEW YORK (USA)
6600093H - 97 DEFIBRILLATOR PADS - 1 PER PK, Pad Size in Inches (L x W) ; 4.7 x 6.1 in / 11.9 x 15.6 cm, Integrated Leadwires ; 60 in / 1.5 M, Backing Material ; Polyethylene Foam, Conductive Element Material ; Tin Copper, Adapter Connector ; Comparable to Physio OEM, Reference Number ; T100AC-PHYSIO, Leads In or Out of Package ; Inside Package, Xray Translucence : Radiolucent, ...
Manufactured by:Nissha Medical Technologies, Inc. based inBuffalo, NEW YORK (USA)
Pad Size in Inches (L x W): 4.6 x 4.8 in / 11.7 x 12.2 cm. Integrated Leadwires:52 in / 1.3 M. Backing Material:Polyethylene Foam. Conductive Element Material:Tin Copper. Adapter Connector:Comparable to Cardiac Science OEM. Reference Number:T100CS. Leads In or Out of Package:Outside Package. Xray Translucence:Radiolucent. Cross Reference::D326 and ...
Manufactured by:Nissha Medical Technologies, Inc. based inBuffalo, NEW YORK (USA)
6600095H - 97 DEFIBRILLATOR PADS - 1 PER PK. Pad Size in Inches (L x W) ; 4.7 x 6.1 in / 11.9 x 15.6 cm. Integrated Leadwires ;60 in / 1.5 M. Backing Material ; Polyethylene Foam. Conductive Element Material ; Tin Copper. Adapter Connector ; Comparable to Zoll OEM. ...
Manufactured by:Nissha Medical Technologies, Inc. based inBuffalo, NEW YORK (USA)
6600102H - 97 DEFIBRILLATOR PADS - 1 PER PK. Pad Size in Inches (L x W) ; 4.7 x 6.1 in / 11.9 x 15.6 cm. Integrated Leadwires ; 60 in / 1.5 M. Backing Material ; Polyethylene Foam. Conductive Element Material ; Tin Copper. Adapter Connector ; Comparable to Zoll OEM. ...
Manufactured by:Jarvik Heart, Inc. based inNew York, NEW YORK (USA)
Some heart failure patients face an increased risk of fibrillation, a dangerously rapid and uncoordinated heartbeat that causes sudden death unless interrupted right away. An implanted cardioverter defibrillator monitors the heartbeat for such irregularities and, should one begin to occur, delivers an immediate electrical counter-shock to restore the heart’s normal ...
Manufactured by:Netech Corporation based inFarmingdale, NEW YORK (USA)
The high adaptability of the device makes it capable of defibrillator testing, transcutaneous pacemaker analysis, and 12-Lead ECG/arrhythmia simulations. You can also routinely track your devices’ performance overtime. Test results can be conveniently saved in the internal memory, uploaded to your PC, and printed using ProComPrint software. ...
Manufactured by:Allegro Industries based inPiedmont, SOUTH CAROLINA (USA)
This white Defibrillator Wall Case is made of corrosion-resistant ABS plastic and includes high visibility defibrillator graphics. It also features a 6″ x 10″ window and right hand door opening. Three optional slide in shelves are also available. ...
by:SimVS based inGLENMONT, NEW YORK (USA)
40 Scenario Library ( 20 ACLS/PALS Scenarios, 20 Nursing Med-Surg ), 2 Tablets: Instructor, Student, Router, Defibrillator ...
by:SimVS based inGLENMONT, NEW YORK (USA)
20 pre-programmed scenarios, Included tablets: 2 (instructor & student), Router, Defibrillator ...
by:SimVS based inGLENMONT, NEW YORK (USA)
SimVS Hospital Virtual Simulation System is a tablet based clinical education solution incorporating a variety of monitor types to create highly realistic and immersive scenarios. SimVS provides monitoring solutions across all specialties and can be used with SP’s, manikins, or full body simulators. ...
by:SimVS based inGLENMONT, NEW YORK (USA)
SimVS Hospital Virtual Simulation System is a tablet based clinical education solution incorporating a variety of monitor types to create highly realistic and immersive scenarios. SimVS provides monitoring solutions across all specialties and can be used with SP’s, manikins, or full body simulators. ...
Manufactured by:Nasco Healthcare Inc. based inSaugerties, NEW YORK (USA)
Developed in cooperation with Weinmann Emergency Medical Technology, the qubeMS2 ventilation simulator is based on MEDUMAT Standard2 used by emergency services. Customizable to support medical device modes with optional add-on medical device software ...
Manufactured by:PAVmed Inc. based inNew York, NEW YORK (USA)
The intraosseous route provides a means for infusing fluids, medications, and other substances directly into the bone marrow cavity which communicates with the central venous circulation via nutrient and emissary veins. This route, which has been used for decades, is well established and has been shown to bioequivalent to the intravenous route. PortIO is a novel, implantable, intraosseous ...