- Home
- Equipment
- usa new york
- hiv 1 hiv 2
Refine by
Hiv 1 Hiv 2 Equipment Supplied In Usa New York
6 equipment items found
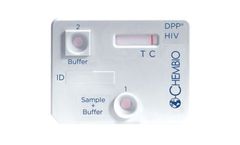
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
An FDA approved, rapid point-of-care test that detects antibodies to HIV-1 and HIV-2. ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
An FDA Approved, dual rapid test for the detection of antibodies to HIV 1/2 and Treponema pallidum in fingerstick whole blood, venous whole blood, or plasma specimens. ...
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
This product contains Human Allograft Material (referred to as “Graft”) collected from human cadaveric donors. All tissue processed for Impladent Ltd. is recovered by U.S. tissue banks as well as a completed donor chart for the enclosed product including but not limited to: serology results, preprocessing culture results, medical and social history evaluation and serodilution ...
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
This product contains Human Allograft Material (referred to as “Graft”) collected from human cadaveric donors. All tissue processed for Impladent Ltd. is recovered by U.S. tissue banks as well as a completed donor chart for the enclosed product including but not limited to: serology results, preprocessing culture results, medical and social history evaluation and serodilution ...
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
A donor serum sample is tested by a CLIA Certified Lab using FDA licensed screening tests and found to be negative or non-reactive for antibodies to human immunodeficiency virus type 1 and type 2 (anti-HIV 1 and anti-HIV 2), hepatitis B surface antigen (HbsAg), hepatitis B core antibody (HbcAb), ...
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
This product contains Human Allograft Material (referred to as “Graft”) collected from human cadaveric donors. ll tissue processed for Impladent Ltd. is recovered by U.S. tissue banks as well as a completed donor chart for the enclosed product including but not limited to: serology results, preprocessing culture results, medical and social history evaluation and serodilution ...