- Home
- Equipment
- usa new york
- medical device directive
Refine by
Medical Device Directive Equipment Supplied In Usa New York
3 equipment items found
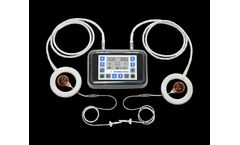
by:Sterling Medical Devices based inMoonachie, NEW YORK (USA)
All medical devices sold in the EU must have a Clinical Evaluation Report (CER) as part of its technical documentation to satisfy the new EU Medical Device Regulation (MDR) requirements. A comprehensive analysis of pre- and post-market data relevant to a medical device, a Clinical Evaluation Report demonstrates that your device achieves its intended purpose without exposing users and patients to ...
Manufactured by:Tuttnauer based inBreda, NETHERLANDS
Medium size chamber in a mobile autoclave. A free-standing, cabinet enclosed unit is simple and as easy to use as any tabletop autoclave. The 3870 HSG is a hospital grade Class B autoclave, suitable for large medical & dental clinics, and operating rooms. Designed to be a fast cycle sterilizer that is mobile, operates 24 hours a day, and can optionally operate independent of building ...
Manufactured by:Avery Biomedical Devices, Inc. based inCommack, NEW YORK (USA)
The Avery Diaphragm Pacing System can provide ventilatory support for patients with chronic respiratory insufficiency whose diaphragm, lungs, and phrenic nerves have residual function. Typically, these patients have high spinal cord injuries, central sleep apnea or other central neurological disorders or a paralyzed diaphragm. A diaphragm pacing system consists of surgically implanted receivers ...