- Home
- Equipment
- usa pennsylvania
- antigen presenting
Refine by
Antigen Presenting Equipment Supplied In Usa Pennsylvania
6 equipment items found
Manufactured by:BriaCell Therapeutics Corp. based inWest Vancouver, BRITISH COLUMBIA (CANADA)
We believe that Bria-IMT™/Bria-OTS™, activates the patient’s immune system to recognize tumor cells and destroy them. We hypothesize that Bria-IMT™/Bria-OTS™, exert their action by stimulating the antigen-presentation system (i.e. the system that presents antigen material on the surface of the tumor ...
Manufactured by:LAVA Therapeutics N.V. based inUtrecht, NETHERLANDS
Vγ9Vδ2 T cells belong to the first line of defense against cancer and have the potential to elicit deep and durable responses in the ...
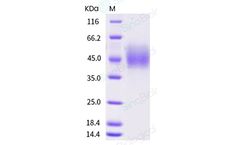
Manufactured by:Sino Biological Inc. based inWayne, PENNSYLVANIA (USA)
Purity:> 97 % as determined by SDS-PAGE. Endotoxin: < 1.0 EU per μg of the protein as determined by the LAL method. ...
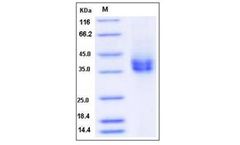
Manufactured by:Sino Biological Inc. based inWayne, PENNSYLVANIA (USA)
FCAR, also called FcαRI or CD89, is a type I transmembrane receptor for Fc region of IgA which is the most abundant immunoglobulin in mucosal areas but is only the second most common antibody isotype in serum. This receptor is present on the surface of myeloid lineage cells such as neutrophils, monocytes, macrophages, and eosinophils, especially phagocytes located in mucosal ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...