- Home
- Equipment
- usa pennsylvania
- biomedical
Refine by
Biomedical Equipment Supplied In Usa Pennsylvania
26 equipment items found
Manufactured by:Amphenol Advanced Sensors based inSt. Marys, PENNSYLVANIA (USA)
Thermometrics Type MA Biomedical Chip Thermistor Assemblies are designed for use in applications involving both intermittent and continuous patient temperature monitoring. Repeatability and fast response are essential, not only for the intermittent temperature requirements associated with oral and rectal fever measurements, but also for the continuous monitoring often necessary ...
Manufactured by:Applied Test Systems based inButler, PENNSYLVANIA (USA)
Breakthroughs in modern spine research and the development of new therapeutic and curative techniques and technologies require an efficient and biomechanically accurate means of obtaining reliable test data.To meet this challenge, Applied Test Systems (ATS) has developed the Series FS20 Biomechanical Spine Test System, capable of performing flexion/extension, lateral bending, and torsion testing ...
Manufactured by:Applied Test Systems based inButler, PENNSYLVANIA (USA)
Breakthroughs in modern spine research and the development of new therapeutic and curative techniques and technologies require an efficient and biomechanically accurate means of obtaining reliable test data.To meet this challenge, Applied Test Systems (ATS) has developed the ST21 and the ST21-TT Biomechanical Spine Test System, capable of performing flexion/extension, lateral bending, and torsion ...
Manufactured by:Shifa Biomedical Corporation based inMalvern, PENNSYLVANIA (USA)
LDLR is a cell-surface receptor that binds LDL-C and carries it into cells to the endosome for degradation and thus reduces the plasma level of LDL-C (Figure 1). However, PCSK9 controls the degradation of the LDLR in the liver (Figure 1) and thereby contributes to cholesterol ...
Manufactured by:Adhezion Biomedical, LLC based inWyomissing, PENNSYLVANIA (USA)
FloraSeal® Microbial Sealant is a sterile, liquid microbial sealant intended for use after topical operative skin preparations with standard surgical draping and prior to a surgical incision. The product is used to reduce the risk of skin flora contamination throughout a surgical ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
BioSphere Flex Sp Extremities is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere® Technology, BioSphere® Flex SP Extremities is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate carrier. ...
Manufactured by:Saladax Biomedical, Inc. based inBethlehem, PENNSYLVANIA (USA)
Neutropenia and neuropathy can be serious side-effects associated with paclitaxel chemotherapy. While hematological toxicities can be addressed, there is no good solution for chemotherapy induced peripheral neuropathy, which is frequent, may be debilitating and can persist or appear after ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
BioSphere Flex SP is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere® Technology, BioSphere® Flex SP is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate carrier. ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
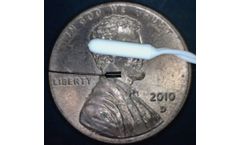
BioSphere Putty is based on an innovative form of 45S5 bioactive glass. The Putty utilizes Synergy's unique spherical bioactive glass particles with a optimized, bimodal size range. The combination of the BioSphere particles and a resorbable, phospholipid carrier results in a bioactive Putty with the highest bioactive glass content on the market, excellent handling, and improved bone ...
Manufactured by:Adhezion Biomedical, LLC based inWyomissing, PENNSYLVANIA (USA)
The most versatile care and maintenance device in vascular access. Standard of care in vascular access typically involves the use of multiple products for securement and insertion site protection. See how SecurePortIV® Catheter Securement Adhesive is combining multiple patient and caregiver benefits into one ...
Manufactured by:Adhezion Biomedical, LLC based inWyomissing, PENNSYLVANIA (USA)
Introducing a gentle giant in surgical and wound closure. SURGISEAL VET™ and SURGISEAL VET™ PRO Topical Skin Adhesives are fast alternatives to sutures. They offer all the benefits of a traditional skin adhesive and ...
Manufactured by:Saladax Biomedical, Inc. based inBethlehem, PENNSYLVANIA (USA)
BSA dosing results in sub-target 5-FU levels in the majority of patients. PK- guided dosing can move 5-FU levels into the target range. Optimal dosing of 5-FU is associated with increased efficacy and reduced ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
BioSphere Flex is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere Technology, BioSphere Flex is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
Data Driven Performance - In Vivo Study Results; Bioactive glass has typically been used in a particulate form consisting of irregular particles and a broad size range (32-710 um). Instead of creating a bone graft with these "off-the-shelf" particles, Synergy utilized a unique spherical particle shape and conducted an in vivo optimization study to determine the size range that provided the best ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
The 45S5 formulation of bioactive glass has been the primary material used in today's bioactive glass bone graft products (45% SiO2, 24.5% CaO, 24.5% Na2O, and 6% P2O5). ...
Manufactured by:Adhezion Biomedical, LLC based inWyomissing, PENNSYLVANIA (USA)
SURGISEAL Topical Skin Adhesive provides the optimal balance between strength and flexibility. SURGISEAL® adhesive can replace sutures for incision or laceration repair and is designed to save time during wound repair, provide a flexible, water-resistant, protective coating, and eliminate the need for suture ...
Manufactured by:Saladax Biomedical, Inc. based inBethlehem, PENNSYLVANIA (USA)
The MyCare Insite Analyser: Valuable Point Of Care Testing For Today’s Psychiatry. MyCare Insite offers greater confidence to clinicians by providing objective data about patient’s medication adherence and efficacy. The Insite measures the blood levels of the most common antipsychotic drugs along with frequently tested HbA1c. Similar to a blood glucose test, Insite requires only a ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
The BioSphere® MIS Putty graft delivery system is precision engineered to extrude BioSphere® Putty through a cannula designed for minimally invasive surgery ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
The BioSphere MIS II Putty graft delivery system is precision engineered to extrude BioSphere® Putty through a cannula designed for minimally invasive surgery ...
Manufactured by:Saladax Biomedical, Inc. based inBethlehem, PENNSYLVANIA (USA)
Targeted therapies are revolutionizing oncology. Patients with chronic myeloid leukemia treated with the tyrosine kinase inhibitors (TKI) now have life expectancies approaching that of the general population. Imatinib (Gleevec®, Gilvec®) the first TKI approved has substantial efficacy in chronic myeloid leukemia (CML) and gastrointestinal stromal ...