- Home
- Equipment
- usa pennsylvania
- bone graft
Show results for
Refine by
Bone Graft Equipment Supplied In Usa Pennsylvania
15 equipment items found
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
BioSphere Flex SP is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere® Technology, BioSphere® Flex SP is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate carrier. ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
BioSphere Flex Sp Extremities is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere® Technology, BioSphere® Flex SP Extremities is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate carrier. ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
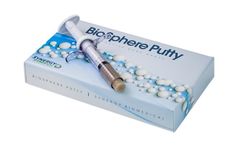
BioSphere Putty is based on an innovative form of 45S5 bioactive glass. The Putty utilizes Synergy's unique spherical bioactive glass particles with a optimized, bimodal size range. The combination of the BioSphere particles and a resorbable, phospholipid carrier results in a bioactive Putty with the highest bioactive glass content on the market, excellent handling, and improved bone ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
BioSphere Flex is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere Technology, BioSphere Flex is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
The 45S5 formulation of bioactive glass has been the primary material used in today's bioactive glass bone graft products (45% SiO2, 24.5% CaO, 24.5% Na2O, and 6% P2O5). ...
Manufactured by:Omnia LLC based inAbbottstown, PENNSYLVANIA (USA)
Omnia surgical PTFE sutures are ideal for any implant, periodontal and bone graft procedures where the usage of a monofilament suture with low bacterial adhesion is recommended. Omnia PTFE sutures are soft, biologically inert and chemically non reactive. Compared to other monofilament synthetic sutures, PTFE sutures are well-tolerated in the oral ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
Data Driven Performance - In Vivo Study Results; Bioactive glass has typically been used in a particulate form consisting of irregular particles and a broad size range (32-710 um). Instead of creating a bone graft with these "off-the-shelf" particles, Synergy utilized a unique spherical particle shape and conducted an in vivo optimization study to determine the ...
Manufactured by:Omnia LLC based inAbbottstown, PENNSYLVANIA (USA)
Omnia surgical PTFE sutures are ideal for any implant, periodontal and bone graft procedures where the usage of a monofilament suture with low bacterial adhesion is recommended. Omnia PTFE sutures are soft, biologically inert and chemically non reactive. Compared to other monofilament synthetic sutures, PTFE sutures are well-tolerated in the oral ...
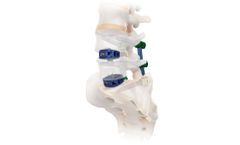
Manufactured by:Camber Spine Technologies, LLC based inKing of Prussia, PENNSYLVANIA (USA)
The SPIRA -C Integrated Fixation System consists of a stand-alone interbody fusion device with internal screw fixation, for one or two levels from the C2-C3 disc to the C7-T1 ...
Manufactured by:Camber Spine Technologies, LLC based inKing of Prussia, PENNSYLVANIA (USA)
All titanium, 3D-printed construct with inferior and superior surfaces specifically designed with a controlled, trabecular pattern to create an environment for fusion to ...
Manufactured by:Centinel Spine based inWest Chester, PENNSYLVANIA (USA)
3D-printed porous titanium lumbar interbody with 2-level ...
Manufactured by:Centinel Spine based inWest Chester, PENNSYLVANIA (USA)
3D-printed porous titanium cervical interbody with 2-level clearance! lexible: FLX implants include a solid exoskeleton for added strength, along with a proprietary lattice scaffold designed to have a stiffness similar to bone. Reduced device stiffness is known to minimize stress-shielding and enhance the fusion process. Lucent: FLX implants include porous radiolucent sections with optimized ...
Manufactured by:Avanti Orthopaedics, LLC based inPenn Valley, PENNSYLVANIA (USA)
The Avanti Wrist & Forearm System contains meticulously designed and manufactured implants to address the variety of wrist pathology encountered by today’s surgeons. The Spanning and Fusion plates are very low profile to facilitate application and reduce soft tissue complications. Far-cortical locking screws reduce construct rigidity to reduce stress shielding and promote rapid bone ...
Manufactured by:A. Titan Instruments based inOrchard Park, NEW YORK (USA)
The Mixing Bowl is made from stainless steel has a weighted bottom to prevent tipping or spilling while mixing bone and other grafting ...
Manufactured by:Stryker based inKalamazoo, MICHIGAN (USA)
Conveniently collects drilled bone dust for use as autograft material during ortho/spine, cranial, otology or other procedures where bone regeneration is ...