- Home
- Equipment
- usa tennessee
- clinical validation
Refine by
Clinical Validation Equipment Supplied In Usa Tennessee
4 equipment items found
Manufactured by:Embra Medical LLC based inJackson, TENNESSEE (USA)
The Embra E-12 ECG/EKG Machine boasts exceptional cost performance with both accurate clinical performance as well as user-friendly design. It is equipped with a comprehensive Alphanumeric keyboard as well as a practical one-touch operation with both age and gender ...
Manufactured by:Insightra Medical Inc, a Mundomedis Group Company based inClarksville, TENNESSEE (USA)
Through better design, the Insightra Ultra 7Fr catheter allows common fluid-filled technology in a smaller catheter with no compromise on lumen size. This ULTRA 7FR IAB catheter is a true 7Fr (including the wrapped balloon), so it will pass through all 7Fr sheaths. 7Fr means a 23% reduction on cross-sectional area (vs. a competitive 8Fr), which results in over 20% better distal blood flow in a ...
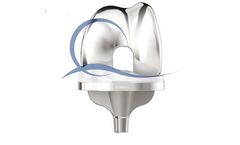
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
Patient expectations are not as well fulfilled by TKA as by total hip replacement with fewer knee patients achieving a “forgotten joint” replacement. Studies show that around 20% of TKA patients are not satisfied[1, 2, 3]. Excessive A/P motion may result in anterior knee pain and continued swelling. In many P/S designs, the stabilizing mechanism only engages after 70-80° of ...
Manufactured by:DT MedTech LLC based inMcMinnville, TENNESSEE (USA)
The Hintermann Series H2 Total Ankle Replacement System (Hintermann Series H2) is a semi-constrained, total ankle replacement system consisting of a talar component, tibial assembly component, and a PE inlay assembly component. Prof. Beat Hintermann developed the Hintermann Series H2 based on his more than 20 years of experience implanting his successful Hintermann Series H3® ankle outside ...