Refine by
Allograft Equipment Supplied In Usa Texas
19 equipment items found
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
Coll-e-Strong allografts are Parametrics Medical’s trademarked electron beam sterilized tendons. This innovative processing methodology achieves a sterility assurance level (SAL) of 10-6 without the use of gamma irradiation. Coll-e-Strong grafts provide Parametrics Medical’s best option of sterility, safety, and strength for surgeons and patients. ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The Trinity ELITE allograft, a third generation allograft with viable cells, provides a unique alternative to autograft, long considered the standard for bone grafting. Exclusively processed for Orthofix by our premier partner, MTF Biologics, the Trinity ELITE allograft builds on the exemplary safety profile of more than 200,000 Trinity Evolution ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The Trinity Evolution allograft is comprised of cancellous bone with viable osteogenic and osteoprogenitor cells retained within the matrix and a demineralized cortical bone component. The Trinity Evolution allograft offers an ideal alternative to autograft and other bone grafting ...
Manufactured by:SurgiLogix based inSan Antonio, TEXAS (USA)
SXDBM is an advanced healing technology that is revolutionizing the way doctors approach bone defects. This unique human allograft tissue is derived from human bone which has undergone a specialized demineralization process that maintains the bone’s natural growth factors. When implanted into a bone void or gap, SXDBM™ bone allograft has the potential ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
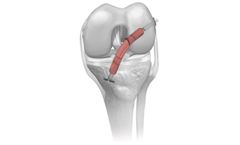
The biomechanical role of the meniscus is load distribution and stability in the knee joint. Parametrics Medical’s allograft meniscus with hemi plateau bone block is used in meniscal allograft transplant procedures and one of the few viable treatment options for the young meniscectomized knee. Our allograft meniscus are aseptically ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
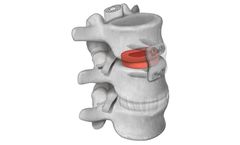
Machined interbody spacers provide a natural osteoconductive option for spine fusion. The allograft bone is precision machined to provide an optimal fit and stability between the allograft and adjacent vertebral bodies. Parametrics Medical offers spacers in various profiles, footprints, and ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
OsteoSource™ allograft bone particulates have osteoconductive properties and are well suited to fill bone structure defects. Osteoconductive allografts act as a scaffold for the growth of natural bone. Over time, the natural bone replaces the donor’s bone. ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
ACTIGEN™ Verified Inductive Bone Matrix is a naturally derived, differentiated allograft matrix with robust handling ...
Manufactured by:Wenzel Spine, Inc. based inAustin, TEXAS (USA)
VariLift®-LX is the only posterior stand-alone expandable lumbar interbody fusion device cleared by the FDA for 1 or 2 levels, PLIF or TLIF, with or without supplemental fixation, and intended for use with autograft and/or allograft tissue. ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
Demineralized bone matrix (DBM) is decalcified allograft bone with osteoinductive potential. The demineralization process exposes growth factors, such as bone morphogenetic proteins (BMP), trapped by minerals. Growth factors recruit immature cells and stimulate those cells to develop into active bone-forming cells called ...
Manufactured by:Axogen (AXGN) based inAlachua, TEXAS (USA)
Avance Nerve Graft is an off-the-shelf processed human nerve allograft intended for the surgical repair of peripheral nerve discontinuities. Through a proprietary cleansing process for recovered human peripheral nerve tissue, the graft preserves the essential inherent structure of the ECM while cleansing away cellular and noncellular ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
EnduriFuse is allograft bone containing viable bone-derived cells. This innovative graft contains properties ideal for the growth of natural bone: An osteoconductive three-dimensional scaffold with cortical and cancellous components. A demineralized bone scaffold with osteoinductive potential containing growth factors that recruit immature cells and stimulate those cells to ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
AmnioStrip is comprised of placental tissue allowing it to be effective at protecting a wide variety of wounds, while simultaneously creating an environment conducive to the regeneration of healthy tissue. Versatile tissue with application in a variety of surgical procedures. Literature has documented amnion contains collagen, has a variety of growth factors, is compatible and absorbs within the ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The VersaShield amniotic membrane is designed to serve as a wound covering and protective barrier for a variety of surgical ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Promote OsteoStrip is a pliable and absorbable cancellous bone graft that also exhibits osteoconductive and osteoinductive properties. These characteristics used as a homologous tool for bone grafting in spinal fusions, non-unions, and bone fractures. OsteoStrip is processed using a next generation proprietary processing method that maintains the interconnected structure of trabecular ...
Manufactured by:SurgiLogix based inSan Antonio, TEXAS (USA)
Minimally-Manipulated Amniotic Membrane: SXBarrier is comprised of minimally manipulated amniotic membrane derived from the placental tissue of consenting mothers at the time of a live, full-term, elective Cesarean birth. We utilize a specialized processing technique that results in a graft that maintains the membrane’s original biologic and physiologic properties to create a biological and ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Covered by Medicare, Prospera is a transplant rejection assessment test that uses a simple blood draw to evaluate the risk of rejection of a transplanted kidney. Through the use of advanced cell-free DNA technology, Prospera increases a provider’s ability to identify otherwise undetected rejection that might lead to kidney loss. Catching transplant rejection as soon as possible can help ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The CONSTRUX Mini PEEK Spacer System is comprised of a variety of implants manufactured from PEEK in two footprint sizes, small and large, and in various heights, in one-millimeter increments. The superior and inferior surfaces of the implant have a pattern of ripples to provide increased stability and help prevent anterior/posterior movement of the ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
Synthetic bone void fillers are safe, biocompatible, and easy to use. These resorbable biomaterials are based on nano-crystalline hydroxyapatite (HA) and purified collagen technologies. These products are engineered to optimize the resorption rate. Our granules are composed of 60% HA and 40% β-Tricalcium Phosphate (TCP). The formulation provides optimal osteoconduction, and the composition ...