Refine by
Automated Sound Processing Equipment Supplied In Usa Texas
6 equipment items found
Manufactured by:InGeneron, Inc. based inHouston, TEXAS (USA)
InGeneron’s Transpose® Ultra Tissue Processing Unit, in conjunction with the products of the Transpose® Ultra System, enables point-of-care rapid isolation of regenerative cells. It is an automated and preprogrammed processing device for the heating, agitation, and centrifugation of adipose-derived tissue. Up to four samples can be processed at the same time. Both regenerative cells ...
by:Accruent based inAustin, TEXAS (USA)
Manage attributes of all network connected medical devices. Conduct risk assessments, reconcile MDS2 data and other security attributes against inventory, and remediate potential risks and vulnerabilities. Automate workflows unique to your ...
Manufactured by:Zimed Healthcare Inc. based inHouston, TEXAS (USA)
Automated Gram Stainer ZGS-A10 is an automatic device providing precise and standardized results with sample tray capacity of up to 24 pcs. Equipped with an electromagnetic lock with automatic opening and an indicator light on the panel to show the working status of the machine for the convenience of the operator. Designed with self-regulated dyeing process, requiring only loading of slides to ...
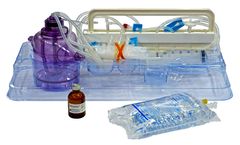
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
The Icellator2 system set the standard, providing SVF cell yields in 65 minutes. Currently approved for use in The Bahamas and available in Japan. In the USA, clinical FDA-approved IDE studies using the Icellator® are underway for a variety of ...
Premium
Manufactured by:LAND® based inDronfield, UNITED KINGDOM
Combining LAND’s cutting-edge, non-contact SPOT+ technology with specialised software algorithms, the SPOT+ GS is a high-precision digital pyrometer that delivers reliable temperature readings for galvanized and galvannealed steel ...
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
Sterile, single-use disposable kit using adipose tissue for high efficiency enzyme derived SVF isolation with Icellator2. Functionally closed system. Minimizes process and cell product variability. Temperature controlled digestion and separation. Class III approval for commercial use by the South Korean Ministry of Food and Drug Safety (MFDS). Approved for clinical use in South Korea & The ...