Refine by
Patient Mobility Equipment Supplied In Usa Texas
5 equipment items found
Manufactured by:United Imaging Healthcare Co., Ltd. based inHouston, TEXAS (USA)
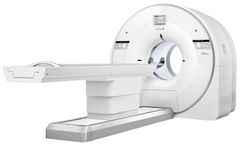
The uMI 550 makes digital PET/CT accessible to all. Built with uEXPLORER PET technology inside, the uMI 550 enables clinical flexibility with digital technology that prevents ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The PhysioStim device provides a safe, non-invasive option for treating fractures that are difficult to heal. With an overall clinical success rate of 80 percent (up to 88 percent for fracture gaps less than 3mm), PhysioStim devices have high success rates for treating nonunion fractures. The device assists in fracture healing by delivering a pulsed electromagnetic field (PEMF) signal to the ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
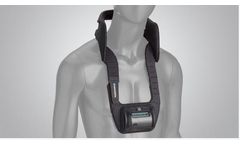
The CervicalStim device is the only bone growth stimulation therapy approved by the FDA as a noninvasive, adjunctive treatment option for cervical fusion in patients at high-risk for non-fusion. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing at a molecular, cellular, and tissue level. With an overall ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
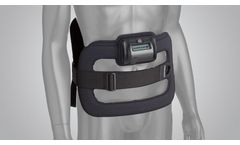
The SpinalStim device is FDA approved to be used after spinal fusion surgery or to be used to treat a failed fusion from a previous surgery. The devices stimulate the natural healing process of bone by sending low-level pulses of electromagnetic energy to the injury or fusion site. The device has an overall clinical success rate of 92% in treating spinal fusion surgery patients. In addition, the ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Renasight is a test to determine if there is a genetic cause for an individual’s kidney disease or if there is an increased hereditary risk due to family history. The test uses a blood or saliva sample to test 385 genes associated with chronic kidney disease (CKD). Results are available in approximately 3 ...