Show results for
Refine by
Spine Surgery Equipment Supplied In Usa Texas
14 equipment items found
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The patient must be skeletally mature, at least 21 years of age, presenting with moderate to severe low back pain (with or without leg pain) that is unresponsive to non-surgical conservative treatments. The patient should not have a history of prior lumbar spine surgery. The patient should have a minimum disc height of 6 mm. ...
Manufactured by:Surgix based inSpring, TEXAS (USA)
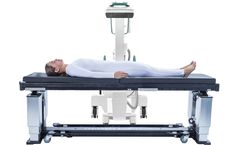
Still juggling multiple tables in your spine room? The FusionMAX®?Elite is a single, reconfigurable table designed to handle both cervical and lumbar spine procedures with ease. Featuring Quick-Change™ radiolucent tops, ultra-light accessories, and a compact footprint perfect for crowded ORs and ASCs, FusionMAX®?Elite reduces setup time, eliminates clutter, and empowers surgical ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Transforaminal lumbar intervertebral device manufactured from PEEK Optima LT1 with tantalum markers for radiographic ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Posterior lumbar intervertebral device manufactured from PEEK Optima® LT1 with tantalum markers for radiographic ...
Manufactured by:Voss Medical Products based inSan Antonio, TEXAS (USA)
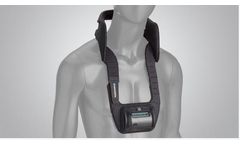
THE VM/S CLH Kit is a safer, cost effective method of shoulder retraction. Use The VM/S Cervical Laminectomy Harness Kit to stabilize the head while retracting the shoulders caudally during anterior and posterior spine operations. It’s cost effective and safer than tape for the surgeon and ...
Manufactured by:Enovis based inLewisville, TEXAS (USA)
CMF Spinalogic is a portable, battery-powered, micro-controlled, noninvasive bone growth stimulator indicated as an adjunct electromagnetic treatment to primary lumbar spinal fusion surgery for one or two ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
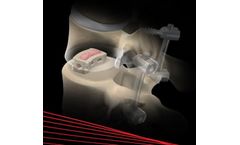
The PerQdisc is designed for use using a lateral traspsoas or retroperitoneal (i.e., anterolateral) surgical approach to access disc space. Access through the annulus can be achieved using the PerQdisc™ Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed through the disc access using various tools to remove most of the ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The CervicalStim device is the only bone growth stimulation therapy approved by the FDA as a noninvasive, adjunctive treatment option for cervical fusion in patients at high-risk for non-fusion. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing at a molecular, cellular, and tissue level. With an overall ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
CellerateRX® Surgical Powder is hydrolyzed collagen that provides a moist environment for surgical sites and wounds. CellerateRX® Surgical Powder is composed of hydrolyzed fragments of Type I Bovine Collagen that are approximately 1/100th the size of native ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
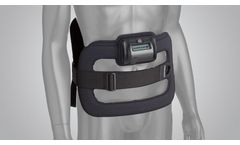
The SpinalStim device is FDA approved to be used after spinal fusion surgery or to be used to treat a failed fusion from a previous surgery. The devices stimulate the natural healing process of bone by sending low-level pulses of electromagnetic energy to the injury or fusion site. The device has an overall clinical success rate of 92% in treating spinal fusion surgery patients. In addition, the ...
Manufactured by:SurgiLogix based inSan Antonio, TEXAS (USA)
SXDBM is an advanced healing technology that is revolutionizing the way doctors approach bone defects. This unique human allograft tissue is derived from human bone which has undergone a specialized demineralization process that maintains the bone’s natural growth factors. When implanted into a bone void or gap, SXDBM™ bone allograft has the potential to induce the full developmental ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
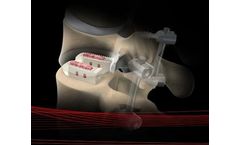
The CONSTRUX Mini PEEK Spacer System is comprised of a variety of implants manufactured from PEEK in two footprint sizes, small and large, and in various heights, in one-millimeter increments. The superior and inferior surfaces of the implant have a pattern of ripples to provide increased stability and help prevent anterior/posterior movement of the ...
Manufactured by:Genesis Medical Plastics based inCypress, TEXAS (USA)
As one of the most complicated structures in the human body, the spinal column must be handled carefully, and with an eye for the future. Surgical intervention to correct conditions like degenerative disc disease (DDD), lumbar spinal stenosis, degenerative scoliosis or degenerative spondylolisthesis is a challenge, and the risks can escalate with time and when additional surgery is required. As ...