Refine by
Validates Important Clinical Equipment Supplied In Usa Texas
11 equipment items found
Manufactured by:Northgate Technologies, Inc. based inElgin, TEXAS (USA)
The NEBULAE® I System is a flexible and cost-effective insufflation system that provides higher clinical performance. The modular design allows the surgeons to enhance their procedure at any given time, alleviating the need to commit to an expensive setup at the onset of the ...
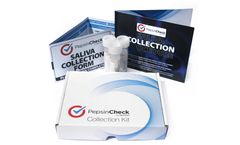
Manufactured by:Restech based inHouston, TEXAS (USA)
PepsinCheck is a simple saliva test for ...
by:Vigilant Biosciences, Inc. based inAustin, TEXAS (USA)
Vigilant Biosciences® product is based on advanced patented technology, including an innovative methodology for assessing multiple biomarkers that, together with other clinical factors, can aid clinicians in the early detection of oral cancer. The clinically validated† test contributes to early and accurate† detection of oral cancer, potentially even before visual or physical ...
Manufactured by:SpeeDx based inSydney, AUSTRALIA
Single well qPCR test for detection of Neisseria gonorrhoeae and markers linked to ciprofloxacin susceptibility validated on a range of specimens and collection vessels. Antibiotic resistance in gonorrhoea infections is a global concern. There are limited effective treatment options and novel antibiotics are not readily available. Smarter and more directed use of antibiotics can be achieved ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Panorama is a blood-based genetic, prenatal screening test of the pregnant person that screens for common chromosomal conditions that affect a baby’s health. Panorama uses SNP*-based technology to deliver highly accurate results and unique insights for both singleton and twin pregnancies. Panorama can be performed as early as nine weeks gestation. Most results will be returned to your ...
by:Castle Biosciences, Inc. based inFriendswood, TEXAS (USA)
Predicting Individual Risk of Metastasis: DecisionDx-SCC is now commercially available. DecisionDx-SCC is clinically validated as a significant predictor of metastasis and the strongest predictor of risk compared to traditional clinical and pathological factors. DecisionDx-SCC complements traditional prognostic risk assessment factors, enabling more informed choices about treatment and follow-up ...
by:Castle Biosciences, Inc. based inFriendswood, TEXAS (USA)
Uveal melanoma, commonly known as ocular or choroidal melanoma, is a rare cancer of the eye with approximately 2,000 patients diagnosed annually in the U.S. At the time of initial diagnosis, approximately 97% of patients are free of metastasis. However, within five years, approximately 30% of patients will develop metastasis – rising to nearly 50% in the following ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Covered by Medicare, Prospera is a transplant rejection assessment test that uses a simple blood draw to evaluate the risk of rejection of a transplanted kidney. Through the use of advanced cell-free DNA technology, Prospera increases a provider’s ability to identify otherwise undetected rejection that might lead to kidney loss. Catching transplant rejection as soon as possible can help ...
Manufactured by:HeartSine Technologies LLC. based in, PENNSYLVANIA (USA)
Designed for simplicity, the compact and lightweight HeartSine samaritan PAD 350P Connected AED offers Wi-Fi connectivity to LIFELINKcentral AED Program Manager, providing key features to help ensure AED ...
Manufactured by:HeartSine Technologies LLC. based in, PENNSYLVANIA (USA)
Featuring CPR Rate Advisor, HeartSine samaritan PAD 450P Connected AED offers real-time feedback on CPR rate as well as key features that help ensure ...
Manufactured by:HeartSine Technologies LLC. based in, PENNSYLVANIA (USA)
Designed for simplicity, the simple to own, fully automatic HeartSine samaritan PAD 360P Connected AED offers Wi-Fi connectivity to LIFELINKcentral AED Program Manager, providing key features to help ensure AED ...