Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Applications
- Diagnostic Solutions for Lung Cancer
- Liquid Biopsy Solutions for Breast Cancer
- Diagnostic Solutions for Colorectal Cancer
- Actionable Molecular Diagnostic System for Physicians
- Vascular Solutions for Dementia Wandering
- MouthLab - Mobile Monitoring System for Biopharmaceutical
- MouthLab - Mobile Monitoring System for Contract Research Organizations (CROs)
- Diagnostic Solutions for Depression
Biomark Equipment Supplied In Usa
252 equipment items found
Manufactured by:COGNISION based inLouisville, KENTUCKY (USA)
COGNISION® is cleared by the FDA. COGNISION® is an objective biomarker system that offers clarity in a field of complexity. By facilitating an objective evaluation of patients with cognitive disorders, COGNISION® helps neurologists and other physicians differentiate between dementia and depression, track disease progression and assess overall cognitive ...
Manufactured by:Imeka based inSherbrooke, QUEBEC (CANADA)
Better Results from Higher Resolution; Any research involving white matter will benefit from our ...
Manufactured by:INOVIQ Ltd based inNotting Hill, AUSTRALIA
INOVIQ has three autoantibody tests in development for early detection of ovarian, breast and lung cancers. BARD1 autoantibody tests measure autoantibodies to variant BARD1 proteins in the blood and use a proprietary cancer-specific algorithm to combine these levels into a cancer score that identifies the presence or absence of a specific cancer. BARD1 autoantibodies reflect the body’s ...
Manufactured by:Owlstone Medical Ltd. based inCambridge, UNITED KINGDOM
The measurement of the volatile organic compounds (VOCs) produced by the body's metabolic activity is a powerful approach for health monitoring and disease detection. Volatile organic compounds (VOCs) are gaseous molecules that can be sampled quickly and non-invasively from breath with Breath Biopsy®. They can originate either from within the body (endogenous VOCs) or from external sources ...
Manufactured by:Nephria Bio based inPortsmouth, NEW HAMPSHIRE (USA)
Our NephroMark™ biomarker tool gives patients and providers the ability to track kidney health and recovery after an AKI ...
by:Sword Diagnostics, Inc. based inCarmel, INDIANA (USA)
Utilizing our proprietary Sword Swift™ Method Development Process and Sword Swift™ Detection Reagents, our scientists can develop sensitive quantitative assays to measure the amounts of free or bound drug in preclinical or clinical samples with high accuracy and ...
by:Reaction Biology Corp. based inMalvern, PENNSYLVANIA (USA)
In collaboration with the bioinformatics firm 4HF Biotech GmbH which is a bioinformatics firm specializing in cancer data mining to discover new anti-cancer drugs, we offer data mining for analysis of the genetic signature of tumor cell lines that are sensitive for a customer drug to reveal potential biomarkers. The Biomarker Analysis is based on the sensitivity ...
Manufactured by:APDM Wearable Technologies Inc. based inPortland, OREGON (USA)
THE OPAL a research-grade wearable sensor built for ultimate ...
Manufactured by:BioSensics LLC based inNewton, MASSACHUSETTS (USA)
BioSensics provides devices and digital technologies for collection and analysis of speech data during site visits and at predefined frequencies in the home ...
Manufactured by:Bluejay Diagnostics, Inc. based inActon, MASSACHUSETTS (USA)
Bluejay Diagnostics is pleased to present Symphony, a near-patient biomarker detection platform. Symphony aims to improve healthcare outcomes by providing quantitative clinical chemistry results, vital for triage and acute care situations. Symphony combines modern advances in interrupted microfluidics, nanotechnology, and enzyme linked immunosorbent assays (ELISA) to enable ...
Manufactured by:Owlstone Medical Ltd. based inCambridge, UNITED KINGDOM
Non-invasive analysis of biomarkers on breath in early detection and precision medicine. Exhaled breath is more than just air, it contains over 1,000 volatile organic compounds (VOCs) as well as microscopic aerosol particles, also known as respiratory droplets, originating from the lungs and airways. ...
Manufactured by:Alphageneron based inCambridge, MASSACHUSETTS (USA)
Our lead clinical stage natural killer (NK) cell therapy, is an autologous platform technology that activates NK cells to kill cancers expressing a unique membrane form of Heat Shock Protein 70 ...
Manufactured by:INOVIQ Ltd based inNotting Hill, AUSTRALIA
INOVIQ’s NETs technology is a sample preparation platform with the potential to revolutionise the performance of liquid biopsy diagnostic assays. The market potential for liquid biopsy applications is estimated at billions of dollars ...
Manufactured by:bioMérieux S.A. based inMarcy l`Etoile, FRANCE
The Astute140 Meter is a bench-top/tabletop analyzer that converts the fluorescent signal from each of the two biomarker assays, TIMP-2 and IGFBP-7, contained within the Test Cartridge into a single numerical result. The NephroCheck Test results are available on the Astute140® Meter in approximately 20 ...
Manufactured by:Epicore Biosystems, Inc. based inCambridge, MASSACHUSETTS (USA)
Epicore Biosystems, in partnership with PepsiCo and Gatorade, has developed a first-of-its-kind personalized performance tracking sweat patch - the Gx Sweat Patch. The Gx Sweat Patch is a skin-like, wearable patch that pairs with an easy-to-use software app to provide real time and personalized recommendations for athletes to optimally hydrate and refuel after exercise. ...
Manufactured by:Life Diagnostics, Inc. based inWest Chester, PENNSYLVANIA (USA)
ELISA kits for detection of skeletal muscle troponin-C, troponin-I, myoglobin and fatty acid binding protein (FABP) in species including mouse, rat, rabbit, dog, pig, and monkey are available, as are the respective purified skeletal muscle biomarkers. Skeletal muscle troponin-I is considered the biomarker of choice for detection of muscle injury because unlike ...
Manufactured by:Proxim Diagnostics Corp. based inSanta Clara, CALIFORNIA (USA)
Cardiac Troponin is a heart attack blood biomarker that is measured in patients who present symptoms such as chest pain upon arrival in an emergency department. Compared to conventional Troponin tests, our high-sensitivity Troponin I (hs-cTnI) assay enables earlier detection and reliable rule-out. ...
Manufactured by:Quanterix Corporation based inBillerica, MASSACHUSETTS (USA)
The Quanterix SR-X™ Ultra-Sensitive Biomarker Detection System is powered by Simoa® bead technology, and offers researchers access to ultra-sensitive biomarker detection capabilities in a compact and affordable system. The SR-X is designed for multiplex detection of up to six analytes per well, with low volume requirements to increase productivity and ...
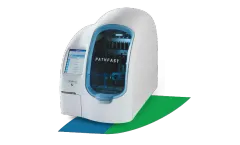
Manufactured by:Polymedco, LLC based inCortlandt Manor, NEW YORK (USA)
The PATHFAST Analyzer is designed to enhance cardiac care in emergency department settings by providing high-sensitivity cardiac troponin (hs-cTn) and NT-proBNP testing capabilities. This compact, benchtop device delivers rapid, lab-quality results within 17 minutes from whole blood samples, utilizing chemiluminescence and Magtration® technology. The system's flexibility allows simultaneous ...
Manufactured by:Bionote based inBig Lake, MINNESOTA (USA)
The first and only rapid, in-clinic canine cardiac biomarker test, Vcheck's cNT-proBNP test allows for identification of this marker to assess NT-proBNP concentration increases secondary to excessive stretching of heart muscle cells, allowing this marker to be used to assess the severity of underlying heart ...