Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Blood Collection And Interlocked Test Device Equipment Supplied In Usa
22 equipment items found
Manufactured by:Seventh Sense Biosystems Inc. based inMedford, MASSACHUSETTS (USA)
TAP II is available for Investigational Use only and is not yet FDA Cleared. TAP II is currently CE Marked and available in select European countries. TAP II is being evaluated as a blood-collection device with a detachable blood reservoir under development by Seventh Sense ...
Manufactured by:Genesis BPS based inRamsey, NEW JERSEY (USA)
Our Data Collection Mixer stands alone in the field of blood collection technology and redifines the standards for blood collection. ...
Manufactured by:Genesis BPS based inRamsey, NEW JERSEY (USA)
The Genesis Blood Collection Mixer stands alone in the field of blood collection technology – the most accurate, durable and reliable automated mixer. ...
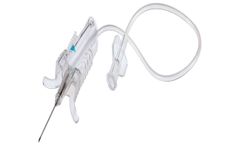
Manufactured by:Retractable Technologies, Inc. (RTI) based inLittle Elm, TEXAS (USA)
Automated in-vein retraction effectively reduces the risk of needlestick injuries during blood collection. The trigger indicator is color coded to easily identify needle gauge, and the design of the finger grips allows for easy handling. When activated, needle retraction clamps the tubing, reducing the risk of exposure to blood. Another unique feature is the clear body, which allows for flashback ...
Manufactured by:Retractable Technologies, Inc. (RTI) based inLittle Elm, TEXAS (USA)
The needle is automatically retracted from the patient when the end-cap is closed after the last tube has been removed. This pre-removal activation virtually eliminates exposure to both ends of the contaminated needle and allows users to keep both hands behind the needle when activating, reducing the risk of needlestick injuries. VanishPoint blood collection tube holders are single-use, adhere to ...
by:RAM Scientific, Inc. based inNashville, TENNESSEE (USA)
RAM Scientific’s SAFE-T-FILL Capillary Blood Collection Tubes are 100% plastic capillary blood collection systems. Available for a wide range of applications, the Capillary Collection Tubes are pre-assembled with a capillary tube, attached cap and a micro ...
Manufactured by:PTS Diagnostics Inc. based inWhitestown, INDIANA (USA)
Capillary Tube Speeds Blood Collection and Ensures Savings. PTS Collect™ capillary tubes provide speed, efficiency and savings - especially for applications where exact sample volumes are required. PTS Collect capillary tubes provide better efficiency and are designed to be easier to use than traditional blood transfer tubes. Using a single glass tube with plastic casing, PTS Collect ...
Manufactured by:DCN Dx based inCarlsbad, CALIFORNIA (USA)
Whether you’re developing a blood collection device or a blood-based LFA, save valuable time with the DCNovations Blood Collection/Separation Materials Kit. This all-in-one kit provides the materials you need for your blood-based project to filter out RBCs without hemolysis. Don’t waste time researching materials, contacting individual suppliers, and coordinating shipments: the Blood ...
Manufactured by:MaxQ Research based inStillwater, OKLAHOMA (USA)
MaxOne is designed as a versatile solution for blood centers seeking reliable transportation of blood products. It revolutionizes inventory management by replacing the need for diverse box sizes and qualifications, providing a singular, streamlined option for donor and hospital services. Engineered to offer consistent and superior thermal performance, MaxOne maintains the integrity of blood ...
Manufactured by:Magnolia Medical Technologies based inSeattle, WASHINGTON (USA)
Steripath Gen2 is the simple, all-in-one solution clinically proven to reduce false positive blood culture results. Reduce your blood culture contamination rate to improve patient safety and key quality outcomes, while significantly reducing unnecessary antibiotic usage, length of stay and hospital ...
Manufactured by:SiO2 Materials Science based inAuburn, ALASKA (USA)
Blood Preservation Best sample integrity, unparalleled preservation, and the best shelf life. SiO2 Materials Science is a company with deep roots in chemistry and engineering. The company creates engineered blood collection tubes for the Genomics & Diagnostics market. Our technology solves significant problems and opens new possibilities for genomic laboratories. The SiO2 blood collection ...
Manufactured by:Terumo Blood and Cell Technologies, Inc. based inLakewood, COLORADO (USA)
Boost productivity, collect higher-quality components and improve the apheresis experience. The NEW Trima Accel 7 includes some exciting improvements to a world-leading apheresis ...
Manufactured by:Tasso, Inc. based inSeattle, WASHINGTON (USA)
The Tasso-SST device delivers whole liquid blood samples prepared without anticoagulation, allowing immediate separation of serum for antibody and other testing. Adults and children can successfully collect blood samples at home without direct interaction at a lab or with a mobile phlebotomist. These devices are currently available for investigational use only ...
Manufactured by:Seventh Sense Biosystems Inc. based inMedford, MASSACHUSETTS (USA)
Patients go to the doctor more than a billion times a year to have their blood drawn through venipuncture or fingerstick, which requires an in-person interaction and typically involves discomfort and anxiety for the ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Sample with Confidence: A used needle can cause a needlestick injury, even if it is only exposed momentarily before being shielded. This can cause anxiety for healthcare professionals and patients alike. By utilising a user-centric design that provides protection once the needle is removed from the vein, Unistik® ShieldLock allows HCPs to draw large volumes of blood with ease and helps to ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Sample with Confidence: A used needle may cause a needlestick injury, even if it is only exposed momentarily before being shielded. This may cause anxiety for healthcare professionals and patients alike. With its one-handed, in-vein activated safety shield, Unistik® ShieldLock Ultra helps to minimise the risk of needlestick injuries and give peace of mind for ...
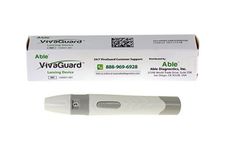
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
Our Lancing Device, used with our Lancets, work together to collect a blood sample for diabetes testing or other blood collection purposes. The Lancing Device can be set to the penetration depth you desire while holding the Lancet securely. Our Lancing Device can be used universally with almost all brands of standard Lancets to collect blood samples used for all other blood glucose monitoring ...
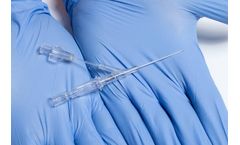
by:Velano Vascular based inSan Francisco, CALIFORNIA (USA)
PIVO is a needle-free, single-use, sterile device that temporarily attaches to a peripheral IV catheter to collect a fresh venous sample. Using the existing PIV line as a conduit to the vein, a flexible, internal flow tube is advanced through the PIV, beyond the catheter tip, and into the vessel to collect a blood sample. This flow tube is designed to extend beyond the suboptimal draw conditions ...
Manufactured by:Lineus Medical based inFayetteville, ARKANSAS (USA)
Drawing Blood Through Single Lumen PIV Catheters Is Sub-Optimal And Increases Risk Of Catheter Failure. For diabetic patients, nurses must obtain a blood sample a minimum of four times a day. When an insulin IV drip is utilized, blood glucose will typically be monitored hourly until it is stable, and usually every two hours after stabilization.(1,2) Even if the patient already has an IV ...
Manufactured by:Streck, Inc. based inLa Vista, NEBRASKA (USA)
Cell-Free DNA BCT is a direct-draw venous whole blood collection device intended for the collection, stabilization and transport of venous whole blood samples for use in conjunction with cell-free DNA next generation sequencing liquid biopsy assays that have been cleared or approved for use with samples collected in the Cell-Free DNA BCT ...