Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Blood Donor Equipment Supplied In Usa
16 equipment items found
Manufactured by:Tempo Medical Products based inPhoenix, ARIZONA (USA)
Lightly textured to provide maximum control; Innovative design prevents tourniquet from slipping or unraveling; Recommended for single patient use, to help reduce the risk of cross-contamination; Used by phlebotomists to initiate blood draws and begin the apheresis process; Ideal product for blood donor and IV starter ...
Manufactured by:Tempo Medical Products based inPhoenix, ARIZONA (USA)
The non latex material will safeguard patients by eliminating latex related allergic reactions; Innovative design prevents tourniquet from slipping or unraveling; Used by phlebotomists to initiate blood draws and begin the apheresis process; Ideal product for blood donor and IV starter kits; Conveniently packed flat for ease of use and simple ...
Manufactured by:Arlington Scientific, Inc. based inSpringville, UTAH (USA)
FDA Cleared For Diagnostic, Blood Donor and Cadaveric Tissue Screening; For over 37 years Arlington Scientific has been a pioneer in delivering new products and technologies to meet the growing demands of clinical diagnostics, blood donor and cadaveric tissue laboratories worldwide. ...
Manufactured by:EKF Diagnostics USA based inBoerne, TEXAS (USA)
The UltraCrit™ Hematocrit Device is a CLIA waived hematocrit device, high accuracy hematocrit measurement for blood donor screening. EKF Diagnostics UltraCrit™Hematocri device is a point-of-care hematocrit device delivering highly accurate measurements for blood donor screening. UltraCrit™Hematocrit Device is a ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ EZ HIV 1/2 is an in vitro RDT for the qualitative detection of antibodies to HIV type-1 and/or type-2 in serum, plasma, capillary whole blood or venous whole blood. The device is a single-use intended for healthcare professional use as an aid to diagnosis only – not for screening blood ...
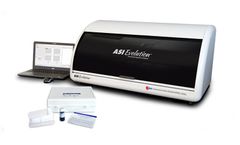
Manufactured by:Arlington Scientific, Inc. based inSpringville, UTAH (USA)
Arlington Scientific’s Automated RPR Syphilis Test is FDA cleared for diagnostic, blood donor screening and cadaveric RPR testing, for use on the ASI Evolution® Automated Syphilis Analyzer. Nontreponemal tests, such as the ASI Automated RPR test, are more reliable indicators of untreated syphilis infections and are used to monitor response to treatment ...
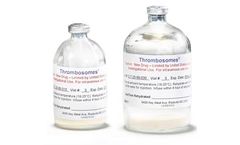
Manufactured by:Cellphire Therapeutics, Inc. based inRockville, MARYLAND (USA)
Thrombosomes are made by pooling platelet units from 10 Group O (Universal) blood donors, removing the plasma and adding a natural sugar, trehalose, to stabilize the cells. Water is then removed through a freeze-drying process resulting in a dry powder. The dry product is heat treated as a final step to reduce the risk of viral transmission and then tested for ...
Manufactured by:Avioq, Inc. based inResearch Triangle Park, NORTH CAROLINA (USA)
The Avioq HIV-1 Microelisa System is intended for use as an aid in diagnosis of infection with HIV-1. It is not intended for use in screening blood donors. Developed as a standard two-step indirect microelisa system assay, the Avioq HIV-1 Microelisa System contains coated 96-well microwell plates, color-coded, liquid calibrator and positive control, and a ...
Manufactured by:Shamrock Labels based inBellwood, ILLINOIS (USA)
Size: 3-1/4" x 4". Unit of Measure: PK. Qty per UOM: 500. Material: Glossy ...
Manufactured by:Immucor based inNorcross, GEORGIA (US) (USA)
Our HLA donor screening assay provides a reliable, accurate and cost effective screening solution. We know your lab has a commitment to keeping blood transfusions safe. Our HLA donor screening assay provides a reliable, accurate and cost-effective screening ...
Manufactured by:Sisu Global Health based inBaltimore, MARYLAND (USA)
The Hemafuse is a handheld, mechanical device for intraoperative autotransfusion of blood collected from an internal hemorrhage, meant to replace or augment donor blood in emergency ...
Manufactured by:Adaptive Biotechnologies based inSeattle, WASHINGTON (USA)
We are currently leveraging our TCR discovery capabilities to enable commercialization of novel therapies by collaborators. In the future, we may explore expanding our end-to-end capabilities for the development of cellular therapies and vaccines. TruTCR is summarized in the figure ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
Mobilized Leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate the migration of CD34+ hematopoietic stem and progenitor cells (HSPCs) from the bone marrow niche into the circulation. Mobilized peripheral blood, enriched in single-donor CD34+ HSPCs is collected through leukapheresis using the ...
Manufactured by:InBios International, Inc based inSeattle, WASHINGTON (USA)
The Chagas DetectTM Plus (CDP) Rapid Test is a rapid immunochromatographic strip assay for the qualitative detection of human lgG antibodies to Trypanosoma cruzi (T. cruzi) in human serum and whole blood matrices (venous and capillary (finger prick) whole blood). CDP is a non-invasive diagnostic test for use in a primary care setting by personnel trained to ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
AllCells’ GMP Fresh Leukopak products are collected by apheresis from peripheral blood of healthy donors. Our Leukopaks contain a high concentration of lymphocyte populations, T Cells, B Cells, NK Cells, and monocytes. AllCells maintains on-site, FDA-registered donor centers for the collection of all products from IRB-consented healthy ...
Manufactured by:Bio-Rad Laboratories, Inc. based inHercules, CALIFORNIA (USA)
The BioPlex 2200 HIV Ag-Ab assay is a multiplex flow immunoassay intended for the simultaneous qualitative detection and differentiation of the individual analytes HIV-1 p24 antigen, HIV-1 (groups M and O) antibodies, and HIV-2 antibodies in human serum or plasma (fresh or frozen K2 EDTA, K3 EDTA, lithium heparin, sodium heparin; fresh citrate). This kit is intended as an aid in the diagnosis of ...