Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Blood Screening Equipment Supplied In Usa
12 equipment items found
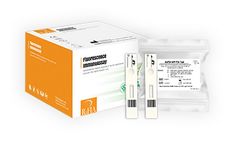
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
AFP’s levels rise in pregnant women, as the liver of the developing fetus produces AFP and it appears in the mother’s blood. Screening during the second trimester of pregnancy is recommended, especially if the mother has a family history of birth defects, is 35 years or older, or has diabetes. Monitoring AFP levels in the mother’s ...
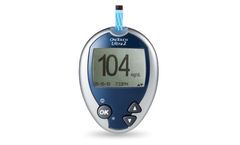
Manufactured by:LifeScan based inMalvern, PENNSYLVANIA (USA)
A fast and simple way to see the effect of food on your blood sugar results. Large screen with backlight. Money Sign; OneTouch® Ultra® test strips have the lowest copay on the most health ...
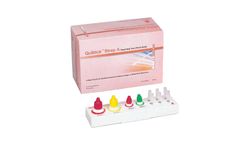
Manufactured by:EKF Diagnostics USA based inBoerne, TEXAS (USA)
QuStick Strep A Rapid Test’s ultra-sensitivity means that it requires fewer colonies to yield a positive result. Which means that even patients presenting less obvious symptoms can be detected. QuStick Strep A Rapid Test from EKF Diagnostics provides qualitative detection of Group A Streptococcal Antigen directly from patient throat swab specimens within five minutes.QuStick Strep A Rapid ...
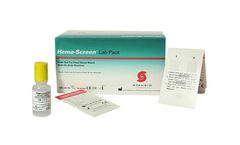
Manufactured by:EKF Diagnostics USA based inBoerne, TEXAS (USA)
Hema-Screen is CLIA waived and uses a guaiac methodology that helps minimize the challenges associated with testing fecal specimens. The cost-effective Hema-Screen® Lab Pack is designed to collect and test specimens for signs of Occult Blood – an early sign of colorectal cancer. The assay is performed in the laboratory and utilizes ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ EZ HIV 1/2 is an in vitro RDT for the qualitative detection of antibodies to HIV type-1 and/or type-2 in serum, plasma, capillary whole blood or venous whole blood. The device is a single-use intended for healthcare professional use as an aid to diagnosis only – not for screening blood ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Panorama is a blood-based genetic, prenatal screening test of the pregnant person that screens for common chromosomal conditions that affect a baby’s health. Panorama uses SNP*-based technology to deliver highly accurate results and unique insights for both singleton and twin pregnancies. ...
Manufactured by:EKF Diagnostics USA based inBoerne, TEXAS (USA)
The UltraCrit™ Hematocrit Device is a CLIA waived hematocrit device, high accuracy hematocrit measurement for blood donor screening. EKF Diagnostics UltraCrit™Hematocri device is a point-of-care hematocrit device delivering highly accurate measurements for blood donor screening. UltraCrit™Hematocrit Device is a ...
Manufactured by:InBios International, Inc based inSeattle, WASHINGTON (USA)
The Chagas DetectTM Plus (CDP) Rapid Test is a rapid immunochromatographic strip assay for the qualitative detection of human lgG antibodies to Trypanosoma cruzi (T. cruzi) in human serum and whole blood matrices (venous and capillary (finger prick) whole blood). CDP is a non-invasive diagnostic test for use in a primary care setting by personnel trained to ...
Manufactured by:Arlington Scientific, Inc. based inSpringville, UTAH (USA)
Arlington Scientific’s Automated RPR Syphilis Test is FDA cleared for diagnostic, blood donor screening and cadaveric RPR testing, for use on the ASI Evolution® Automated Syphilis Analyzer. Nontreponemal tests, such as the ASI Automated RPR test, are more reliable indicators of untreated syphilis infections and are used to monitor response to treatment ...
Manufactured by:Bio-Rad Laboratories, Inc. based inHercules, CALIFORNIA (USA)
The BioPlex 2200 HIV Ag-Ab assay is a multiplex flow immunoassay intended for the simultaneous qualitative detection and differentiation of the individual analytes HIV-1 p24 antigen, HIV-1 (groups M and O) antibodies, and HIV-2 antibodies in human serum or plasma (fresh or frozen K2 EDTA, K3 EDTA, lithium heparin, sodium heparin; fresh citrate). This kit is intended as an aid in the diagnosis of ...
Manufactured by:DiamiR based inMonmouth Junction, NEW JERSEY (USA)
microRNAs are a class of small non-coding regulatory RNA molecules, which modulate target gene expression and protein production, and whose levels often change in disease. Certain microRNAs are enriched, i.e. present at higher concentration, in different brain regions (e.g. hippocampus, midbrain), cells (e.g. neurons), and cellular compartments (e.g. synapses and neurites). microRNAs can cross ...
Manufactured by:Avioq, Inc. based inResearch Triangle Park, NORTH CAROLINA (USA)
The Avioq HIV-1 Microelisa System is intended for use as an aid in diagnosis of infection with HIV-1. It is not intended for use in screening blood donors. Developed as a standard two-step indirect microelisa system assay, the Avioq HIV-1 Microelisa System contains coated 96-well microwell plates, color-coded, liquid calibrator and positive control, and a ...