Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Bone Graft Substitute Equipment Supplied In Usa
42 equipment items found
Manufactured by:Xenco Medical based inSan Diego, CALIFORNIA (USA)
The Sorrento™ Bone Graft Substitute is a robust, resorbable bone void filler to fill voids or gaps of the skeletal system in the extremities and pelvis not intrinsic to the stability of the bony structure. Pre-clinical animal studies have revealed its tremendous performance through early osteoblast activity and osteocyte ...
Manufactured by:NovaBone Products, LLC based inJacksonville, FLORIDA (USA)
NovaBone Putty is a versatile bone graft substitute that is ready to use out of the package with exceptional handling characteristics that will save time and improve placement. The specially formulated consistency means it can be packed into any size or shape void without sticking to surgical gloves. ...
by:Molecular Matrix, Inc. based inWest Sacramento, CALIFORNIA (USA)
Osteo-P™ Bone Graft Substitute (BGS) is a non-mineralized, synthetic bone void filler consisting of a hyper-crosslinked carbohydrate polymer. It is highly porous, biocompatible, biodegradable, and osteoconductive. Osteo-P™ BGS is intended for the filling of bone defects created surgically or as a ...
Manufactured by:Inion Oy based inTampere, FINLAND
Inion BioRestore™ is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore™ is made of degradable bioactive glass, which once in contact with the natural body fluids forms a silica gel and calcium phosphate layer, providing scaffolding for the new bone tissue ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Bonus Synthetic Bone Graft Substitute granules are resorbable, osteoconductive matrices consisting of a thin, 2–10 micron layer of hydroxyapatite over a calcium carbonate core. Bonus Synthetic Bone Graft Substitute has been indicated as a bone graft ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
Synergistic Combination of Bioactive Glass and Demineralized Bone Matrix. Bioactive Matrix is a novel bone graft substitute expressly designed to optimize surgical handling, graft stability and osteoproductivity. ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
βBeta-bsm is injectable osteoconductive material for use in minimally invasive procedures. This synthetic, biocompatible bone graft substitute material sets hard and maintains shape at body temperature. The material is mixed in a closed syringe-to-syringe mixing system to minimize the potential for spills, and can be delivered through a ...
Manufactured by:A. Titan Instruments based inOrchard Park, NEW YORK (USA)
The Mixing Bowl is made from stainless steel has a weighted bottom to prevent tipping or spilling while mixing bone and other grafting ...
Manufactured by:NovaBone Products, LLC based inJacksonville, FLORIDA (USA)
MacroPor Si+ is as a moldable continuous porous structure that can be fitted to the size and shape of the grafting site. The open structure allows for rapid vascularization and mineralization and permits complete device absorption and replacement by new ...
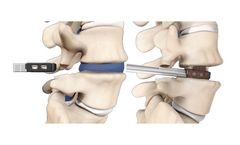
Manufactured by:Precision Spine, Inc. based inParsippany, NEW JERSEY (USA)
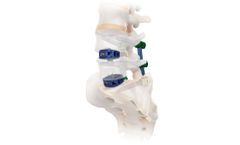
Vault® is a zero profile ALIF system offering midline screw placement to enable easier access, reduced retraction of the neural elements and precise screw placement. The modular plate and cage construct offers two footprints with 8° and 15° lordotic angles to assist in reproducing the patient's sagittal profile while providing anterior column support. The system features four points ...
Manufactured by:SyncMedical LLC based inStoughton, MASSACHUSETTS (USA)
The Ethos C- Spacer is manufactured from PolyEtherEtherKetone (PEEK) with Tantalum markers. It is designed with a flat tipped nose and is available in shorter lengths to properly accommodate the patient’s anatomy. The proud angled tooth on the superior and inferior surfaces was designed to resist migration and large central apertures for placement of bone graft ...
Manufactured by:Spine Wave, Inc. based inShelton, CONNECTICUT (USA)
The Velocity Expandable Interbody Device is a composite, expandable implant that is designed for ease of insertion, optimal implant to patient fit, maximized indirect decompression, and maximum pre and post bone ...
Manufactured by:Isto Biologics based inHopkinton, MASSACHUSETTS (USA)
Fully moldable, 100% demineralized bone graft putty offers optimal regenerative capacity through a proprietary processing of demineralized cortical fibers and demineralized cortical particulate. ...
Manufactured by:Pinnacle Spine Group based inSan Clemente, CALIFORNIA (USA)
InFill Interbody Fusion Systems patented in situ graft delivery solution represents an evolution in addressing the biologic void in spine ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Morpheus and Agilon Moldable bone grafting products offer first-class intraoperative handling, outmatched in the industry. TrelCor granules are suspended in an organic binder, allowing the graft to be molded into the desired shape. The fully absorbable binder is resistant to irrigation which offers excellent graft containment ...
Manufactured by:Camber Spine Technologies, LLC based inKing of Prussia, PENNSYLVANIA (USA)
All titanium, 3D-printed construct with inferior and superior surfaces specifically designed with a controlled, trabecular pattern to create an environment for fusion to ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
One of the primary functions of a bone graft material is to provide an osteoconductive surface for new bone formation. Bone regeneration is a cellular process that involves the interaction of osteoblasts and stem cells with the surface of a bone graft material. Studies have shown that the bone graft surface structure and morphology can impact how cells interact with a material. The TrelCor ...
Manufactured by:Misonix based inFarmingdale, NEW YORK (USA)
You’re used to working with drills and traditional approaches when performing osteotomies. BoneScalpel is an innovative, ultrasonic instrument that puts control in your ...
Manufactured by:Surgentec LLC based inBoca Raton, FLORIDA (USA)
OsteoFlo NanoPutty is the world’s first, and only quadphasic synthetic bone graft putty with nano-surface technology that allows for advanced bone healing. OsteoFlo® NanoPutty® has been designed to provide true nanobiology with an optimal resorption profile and superior handling characteristics. This novel synthetic bone graft putty is comprised of particles featuring four different ...
Manufactured by:Isto Biologics based inHopkinton, MASSACHUSETTS (USA)
InQu® is the cell-friendly biosynthetic™ bone graft with proven clinical efficacy leading to faster bone ...