Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Bone Regeneration Equipment Supplied In Usa
37 equipment items found
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
OsteGro V Osteo Viable Matrix is the Viable Bone Matrix (VBM) product in the OsteGro family. It is comprised of cancellous bone particles with preserved cells combined with demineralized cortical particles and fibers all processed via Aziyo’s proprietary gentle VBM processes. OsteGro Allograft Bone Matrix, the second product in this family, is a sterile bone matrix comprised of cancellous ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
ViBone is comprised of cancellous bone particles with preserved cells and demineralized cortical bone particles produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
Viable Bone Matrix for Spine and Orthopedic Procedures. Fiber Viable Bone Matrix: Fiber Viable Bone Matrix (VBM) is made of cancellous bone particles with preserved cells combined with demineralized cortical fiber produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Osteogenics Biomedical based inLubbock, TEXAS (USA)
Often used with on-lay grafting in ridge augmentation procedures, Cytoplast™ Ti-250 titanium-reinforced membranes provide clinicians with the predictability they are accustomed to with the Cytoplast™ TXT-200 membranes, along with the added benefit of a titanium frame to maintain space during guided bone regeneration. These membranes are also popular ...
Manufactured by:Osteogenics Biomedical based inLubbock, TEXAS (USA)
Often used with on-lay grafting in ridge augmentation procedures, Cytoplast™ Ti-150 titanium-reinforced membranes provide clinicians with the predictability they are accustomed to with the Cytoplast™ TXT-200 membranes, along with the added benefit of a titanium frame to maintain space during guided bone regeneration. These membranes are also popular ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
Over 100 years of research, experimentation and use has demonstrated that calcium sulphate enhances bone regeneration and is totally biocompatible and and resorbable. At the same token, high purity type-I collagen DMBM (xenograft) also is essential for tissue regeneration and remodeling. Osseomold consists of both materials admixed in proper ...
Manufactured by:Stryker based inKalamazoo, MICHIGAN (USA)
Conveniently collects drilled bone dust for use as autograft material during ortho/spine, cranial, otology or other procedures where bone regeneration is ...
by:OSSIO Inc based inWoburn, MASSACHUSETTS (USA)
OSSIOfiber® Cannulated and Solid-Core Fixation Nails allow surgeons to offer patients a strong and biointegrative solution for a wide range of surgical techniques. Comprised of OSSIOfiber® Intelligent Bone Regeneration Technology, Fully integrates into native anatomy without adverse inflammation,, as proven in pre-clinical studies, Rivals performance of ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
One of the primary functions of a bone graft material is to provide an osteoconductive surface for new bone formation. Bone regeneration is a cellular process that involves the interaction of osteoblasts and stem cells with the surface of a bone graft material. Studies have shown that the ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
One of the primary functions of a bone graft material is to provide an osteoconductive surface for new bone formation. Bone regeneration is a cellular process that involves the interaction of osteoblasts and stem cells with the surface of a bone graft material. Studies have shown that the composition of a ...
by:Geistlich Pharma North America Inc. based inPrinceton, NEW YORK (USA)
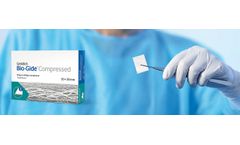
User Benefits: Geistlich Bio-Gide® Compressed is the twin to Geistlich Bio-Gide®, a collagen membrane for reliable bone regeneration and optimal tissue integration.1-3 In comparison, Geistlich Bio-Gide® Compressed has a smoother surface, is firmer in touch and easier to cut.4 Therefore it is especially suitable for dentists who would like alternative ...
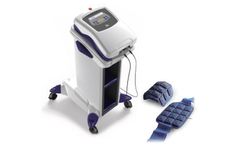
Manufactured by:Cutting Edge Laser Technologies based inRochester, NEW YORK (USA)
ELF electromagnetic fields produce charge displacement, inducing the piezoelectric effect, which is fundamental in the bone regeneration processes. Based on this effect, magnetotherapy can be applied for accelerated healing of delayed union/non-union ...
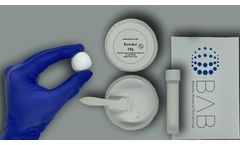
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Cem-Ostetic® is a neutral pH bone putty that contains biocompatible calcium salts that have been used for decades in orthopedic surgery. These materials are often used to provide an additional source of bone to help the patient heal faster. Berkeley Advanced Biomaterials formulates the chemistry and microstructure to enhance bone ...
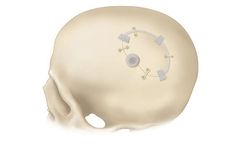
Manufactured by:Bioplate based inPlacentia, CALIFORNIA (USA)
The Osteopore Bioresorbable Bone Scaffold is specifically designed to address craniotomy gaps, promoting improved aesthetics, reduced pain, and enhanced patient satisfaction. Made from polycaprolactone, this biocompatible implant fully resorbs over a span of 18-24 months via in vivo hydrolysis. Its 3D-printed structure mimics cancellous bone, encouraging ...
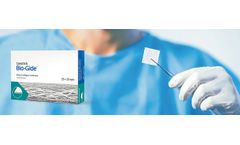
by:Geistlich Pharma North America Inc. based inPrinceton, NEW YORK (USA)
User Benefits: Geistlich Bio-Gide® is a collagen membrane for reliable bone regeneration and optimal tissue integration.1-3. The natural collagen structure of Geistlich Bio-Gide® permits prompt and homogeneous vascularization and so brings about optimal tissue integration and wound stabilization.5 The smooth side of Geistlich Bio-Gide® prevents soft ...
Manufactured by:Himed based inOld Bethpage, NEW YORK (USA)
Himed specializes in high-quality synthetic hydroxyapatite (HA), a calcium phosphate biomaterial known for its biocompatibility and bioactivity, making it highly suitable for bone regeneration, dental reconstruction, and orthopedic implants. Unlike natural forms, Himed's HA is synthetically manufactured to ensure consistent stoichiometry, essential for precise ...
Manufactured by:Himed based inOld Bethpage, NEW YORK (USA)
Beta-tricalcium phosphate (β-TCP) is a calcium phosphate biomaterial noted for its bioactive and resorbable properties, which make it highly effective in applications such as bone grafting and implants. It supports bone regeneration due to its osteoconductive nature, acting as a scaffold for natural bone to replace over ...
Manufactured by:Zetagen Therapeutics Inc. based inSyracuse, NEW YORK (USA)
ZetaMet is a synthetic, small-molecule, inductive biologic technology being developed to target and resolve metastatic bone lesions while inhibiting future tumor growth and regenerating bone. The small molecule has been approved by the U.S. Food and Drug Administration (FDA) since 1971. Zetagen scientists have discovered an entirely new pathway ...
Manufactured by:Augma Biomaterials based inSpotswood, NEW JERSEY (USA)
The Augma Basic Dental Bundle is designed for dental professionals seeking an effective solution in bone grafting and regeneration. It includes six units of Bond Apatite®, a long-term space maintainer that is applicable for various bony defects, provided in 1cc syringes. Additionally, the package contains two units of 3D Bond+™, a short-term space ...
Manufactured by:NuVasive, Inc. based inSan Diego, CALIFORNIA (USA)
An innovative, market-leading solution to help patients with limb length discrepancy (LLD). The Precice system is an intramedullary device that once implanted utilizes an External Remote Controller (ERC) to non-invasively lengthen the femur and ...