Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Applications
- Intraoperative Radiation Therapy (IORT) System - Electron Therapy for Skin Cancer
- Intraoperative Radiation Therapy (IORT) System for Flash Radiotherapy
- Intraoperative Radiation Therapy (IORT) System for Pancreatic Cancer Treatment
- Intraoperative Radiation Therapy (IORT) System for Breast Cancer Treatment
- Intraoperative Radiation Therapy (IORT) System for Single-Fraction APBI
- Intraoperative Radiation Therapy (IORT) System for Surgical Oncologists
- Intraoperative Radiation Therapy (IORT) System for Radiation Oncologists
- Intraoperative Radiation Therapy (IORT) System for Hospital Administrators
Cancer Awareness Month Patient Equipment Supplied In Usa
92 equipment items found
Manufactured by:T-Cure BioScience, Inc. based inSherman Oaks, CALIFORNIA (USA)
Our exclusive iSORT (Isolation of Optimal Reverse Immunology Generated TCR) platform is able to isolate TCRs against unique selected tumor-associated intracellular and extracellular antigens and neoantigens in the context of an HLA molecule, allowing us to focus on specific cancers and patient populations based on their genetic makeups. Using this technology, we are able to isolate TCR ...
Manufactured by:MiraDx based inLos Angeles, CALIFORNIA (USA)
Radiation therapy is a form of cancer treatment that is used in over 2/3 of cancer patients diagnosed every year. Although generally, radiation is a safe treatment, 5-10% of patients will experience serious side effects from treatment. In addition, some patients respond not only locally to radiation, but also have an immune response that can help them to fight their cancer. Miradx is at the ...
by:Calviri based inPhoenix, ARIZONA (USA)
Calviri’s immunotherapy diagnostic is a huge leap forward in predicting therapy outcomes compared to current available ...
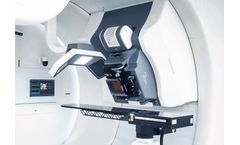
by:IBA based inLouvain-La-Neuve, BELGIUM
Proteus® has been inspired by everyday clinical practice. Through day-to-day interactions with the community, IBA is perfectly positioned to understand, and invest in, the user’s needs. These investments are directly translated into benefits for the patients. The Proteus® design enhances the patient experience by fostering a soothing environment while making the medical ...
Manufactured by:InSilicoTrials Technologies based inStafford, VIRGINIA (USA)
PCa GnRH Agonists Simulator enables simulations of clinical trials on a virtual population of prostate cancer patients being treated with a GnRH agonist ...
by:Acuamark Diagnostics based inNew York, NEW YORK (USA)
The vast majority of cancer cases are detected at stage III or beyond, resulting in poor cancer survival of ~50% in the aggregate. Cancer cases are forecasted to reach 25M per year over the next two decades, causing deaths to rise to 13M per year (worldwide). Annually, the worldwide economic impact of cancer is estimated by the WHO at $1.2 trillion. ...
Manufactured by:Trailhead Biosystems Inc. based inBeachwood, OHIO (USA)
We are developing cell culture media for T-cell subtype control to improve clinical potency in the individual patient and reduction of variability in outcomes between patients. CAR T-cell therapy involves the treatment of cancer by modifying a patient’s T-cells in the lab so they will attack cancer cells. If clinical development of the CART T-cell produces the wrong T-cell subtype, patient ...
Manufactured by:Clarity Diagnostics, LLC based inBoca Raton, FLORIDA (USA)
Clarity understands that early detection of colorectal cancer in patients can be critical for successful treatment. In order to make it easier for your facility to screen for colorectal cancer in-house, Clarity has developed a product that makes the testing process easier and gives you clear results. Introducing Clarity’s One-step Immuno Occult Blood Test Kit or IFOBT for short. Our IFOBT ...
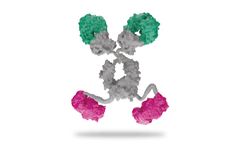
Manufactured by:SystImmune Inc. based inRedmond, WASHINGTON (USA)
By combining bi-valency with bi-specificity in the tetravalent format, the dual checkpoint molecule utilizes avidity and bi-specificity to improve anti-cancer immune cell function. The specificity enhancement both synergistically enhance and expand the breadth of immune cell activity that is diminished in cancer patients (SEBA). The spatio-temporal control of bi-specificity ensure that ...
Manufactured by:InSilicoTrials Technologies based inStafford, VIRGINIA (USA)
A tool that performs in silico clinical trials to assess the neutropenic effects of a chemotherapeutic agent in a virtual population of cancer patients ...
Manufactured by:BioXcel Therapeutics, Inc. based inNew Haven, CONNECTICUT (USA)
BXCL701 (talabostat) is an investigational, oral small molecule inhibitor of dipeptidyl peptidases (DPP)—primarily DPP 8/9 and DPP 4—which triggers inflammasome to alert and prime immune cells, leading to induction of IL-18 and IL-1ß, bridging innate & adaptive immunity. BXCL701 is currently being developed for the treatment of aggressive forms of prostate cancer and ...
Manufactured by:UroGen Pharma, Inc. based inPrinceton, NEW YORK (USA)
UGN-301 is UroGen’s in-licensed anti-CTLA-4 antibody (zalifrelimab). UGN-201 is our proprietary formulation of imiquimod, a toll-like receptor 7 (TLR 7) agonist. When combined, these medicines stimulate both the innate (UGN-201) and adaptive (UGN-301) immune responses, both of which are important for fully harnessing the power of the immune system to fight ...
Manufactured by:Veracyte, Inc. based inSouth San Francisco, CALIFORNIA (USA)
Revealing cancer molecular subtype to inform treatment decisions. Decipher Bladder is a genomic test that measures the molecular profile of bladder cancer using gene expression analysis from transurethral resected bladder tumor specimens. The test was developed for bladder cancer patients with high-grade non-muscle-invasive disease who are being considered for treatment and patients with ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Bladder cancer is the 5th most common cancer in the western world, and 70%-80% of the patients are diagnosed with non-muscle invasive disease. There are estimated 400,000 to 800,000 active bladder cancer patients in the United States, as well as 1 to 2 million in Europe, who undergo local resection of the tumor and then have 1-4 follow-up visits each year due to the high recurrence rate of the ...
Manufactured by:Cancer Targeted Technology based inWoodinville, WASHINGTON (USA)
CTT1057 is an innovative 18F-labeled PET agent for prostate cancer that can be imaged within 2 hours of administration. CTT1057 will specifically and sensitively detect cancer that has escaped from the prostate as well as distant metastatic disease including bone metastases with greater precision and resolution than current standard of care. With uncertainty in how to interpret PSA test results, ...
Manufactured by:Veracyte based inMarseille Cedex 9, FRANCE
Immunoscore® for Rectal Cancer can help identify patients with LARC who are at a low risk of relapse and may be good candidates for organ preservation. Immunoscore® is available through an Innovation Access Program while the company conducts clinical validation ...
Manufactured by:XILIS, Inc. based inDurham, NORTH CAROLINA (USA)
Xilis’ MicroOrganoSphereTM powers the only assay which can provide oncologists with accurate results of drug sensitivity on a patient’s own tumor within the clinical decision-making window - finally realizing the promise of precision medicine. Xilis is developing a panel of disease specific assays systematically testing the most important standard-of-care drugs within equipoise or ...
by:Traws Pharma based inNewtown, PENNSYLVANIA (USA)
Narazaciclib (ON 123300) is a multi-kinase inhibitor targeting CDK 4/6 and additional kinases involved in tumor genesis, which the Company believes presents an innovative approach to treating advanced cancers including HR+ HER2- metastatic breast cancer that is, or has become, resistant to commercial CDK 4/6 inhibitors. Beyond metastatic breast cancer, the Company believes that narazaciclib (ON ...
Manufactured by:Cell Applications, Inc. based inSan Diego, CALIFORNIA (USA)
Mature Human Adipocytes (HAd) are differentiated from HPAd (Human Preadipocytes) through the use of Adipocyte Differentiation Medium. Cells are shipped 5 days after initiating differentiation. Mature HAd are expected 10 days after induction of differentiation and should remain healthy and responsive for at least 2 weeks after complete differentiation. Adipose mass can be controlled by ...
Manufactured by:W.O.M. World of Medicine GmbH based inBerlin, GERMANY
Minimally invasive procedures have become the gold standard worldwide for many indications. Trocars are inserted into the body through small, up to one centimeter long incisions. The surgical teams insert an endoscope and special surgical instruments into the body through these trocars to diagnose and treat conditions. ...