Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Chromatograph Equipment Supplied In Usa
32 equipment items found
by:Wondfo USA Co Ltd. based inWillowbrook, ILLINOIS (USA)
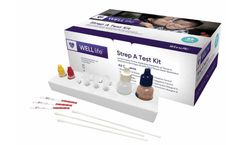
The WELLlife Strep A Test Kit is a rapid chromatographic immunoassay intended for use by healthcare professionals as a qualitative in vitro diagnostic test for detection of group A streptococcal antigen from throat swab ...
Manufactured by:Coretests, Inc. based inCarlsbad, CALIFORNIA (USA)
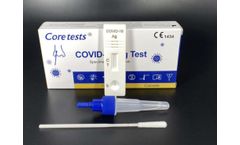
COVID-19 Ag Test is the chromatographic assay test used for qualitative detection of the COVID-19 nucleoprotein antigen in nasal swab specimen from, suspected patients may infected COVID-19. It is intended to be used in self-testing., Specimen:Nasal swab, Detection time at15 minutes, No need for extra equipment, High accuracy, Easy to use, Fast to read ...
Manufactured by:More Diagnostics Inc. based inLos Osos, CALIFORNIA (USA)
More Diagnostics’ Cyclosporine C2 Control has assayed values for Siemens Dimension® and Vista®; Thermo Fisher Cedia® and Siemens Syva EMIT 2000® and is also appropriate for other automated immunoassay systems that correlate with chromatographic ...
Manufactured by:Healgen Scientific, LLC. based inHouston, TEXAS (USA)
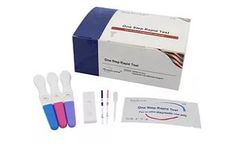
The hCG One Step Pregnancy Test (Urine) is a rapid chromatographic immunoassay for the qualitative detection of human chorionic gonadotropin (hCG) in urine to aid in the early detection of ...
Manufactured by:Ethos Biosciences, Inc. based inLogan Township, NEW JERSEY (USA)
It is a mixture of non-glycated and glycated hemoglobin A0 fractions, but is essentially free of HbA1a, HbA1b, HbA1c and HbF. The protein is validated by HPIEC methods, and is suitable as a primary standard in chromatographic or immunologic analyses. The reported concentration is determined by Drabkins assay. The material is supplied in liquid form in dilute potassium ...
Manufactured by:More Diagnostics Inc. based inLos Osos, CALIFORNIA (USA)
More Diagnostics’ MPA Control has assayed values for Siemens Dimension® MPAT; Siemens Syva EMIT 2000® and LC-MS/MS and is also appropriate for other automated immunoassay systems that correlate with chromatographic ...
Manufactured by:ImmunoVision, Inc. based inSpringdale, ARKANSAS (USA)
Hepatitis B core recombinant antigen isolated from E. coli and purified by chromatographic methodologies. Description: Hepatitis B core antigen (HBcAg) is the major component of the core particles of Hepatitis B Virus (HBV). Particles have a size of 27 nm and contain a circular double-stranded DNA molecule, a specific DNA-polymerase and ...
Manufactured by:More Diagnostics Inc. based inLos Osos, CALIFORNIA (USA)
Assayed Values: Siemens Atellica® Solution, Siemens Dimension® and Vista®; Siemens Syva EMIT 2000®, Abbott Architect® and LC-MS/MS and is also appropriate for other automated immunoassay systems that correlate with chromatographic ...
Manufactured by:BTNX Inc. based inPickering, ONTARIO (CANADA)
The Rapid Response™ Saliva Drug Test (COT) is a lateral flow chromatographic immunoassay for the qualitative detection of COT in oral fluid at the cut-off concentration of 30 ng/mL. This single drug test can provide accurate and fast results in 10 minutes. It is intended for Over-the-Counter Use and is CLIA ...
Manufactured by:Elabscience based inHouston, TEXAS (USA)
COVID-19 (Corona Virus Disease) is an infectious disease caused by the most recently discovered coronavirus. COVID-19 IgG/IgM Rapid Test ( Serum/Plasma/Whole Blood) is a rapid chromatographic immunoassay for the qualitative of IgG and IgM antibodies to COVID-19 in human serum, plasma or whole ...
Manufactured by:Infitek Co., Ltd. based inJinan, CHINA
The Cholera Ag O1 Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of Cholera Ag O1 antigen from fecal samples to aid in the diagnosis of O1 group Cholera ...
Manufactured by:Mountain Horse Solution based inUnion City, TENNESSEE (USA)
The Flowflex COVID-19 Antigen Home Test is a rapid test for the detection of SARS-CoV-2. The lateral flow chromatographic immunoassay is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal swab specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect ...
Manufactured by:Alfa Scientific Designs, Inc based inPoway, CALIFORNIA (USA)
The Instant-view® oral fluid drug test provides faster results at higher accuracy rates with ALFA’s patented Driven Flow® Technology. The test screens up to 14 drugs. It is a one-step, Driven Flow® chromatographic immunoassay based on the principle of competitive binding. Developed & Manufactured in the ...
Manufactured by:Healgen Scientific, LLC. based inHouston, TEXAS (USA)
The EV71 IgM Rapid Test Cassette is a sandwich lateral flow chromatographic immunoassay for the qualitative detection of IgM-class antibodies to human Enterovirus 71 (EV71) in human whole blood, serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
The Antigen Rapid Test assay from the Gold Standard Diagnostics Group is a rapid chromatographic immunoassay for the qualitative detection of SARS-CoV-2 Nucleocapsid (N) Protein antigens present in respiratory ...
Manufactured by:CSL Behring based inKing of Prussia, PENNSYLVANIA (USA)
Albumex 4 is manufactured from human plasma collected by Australian Red Cross Lifeblood. It is prepared using predominantly chromatographic ...
Manufactured by:CSL Behring based inKing of Prussia, PENNSYLVANIA (USA)
Albumex 20 is manufactured from human plasma collected by Australian Red Cross Lifeblood. It is prepared using predominantly chromatographic ...
by:Dynaflow, Inc. based inJessup, MARYLAND (USA)
We have analyzed over 500 water and wastewater samples for PPCP; including over 300 using the USGS 1433 method (other methods can be used or developed for special analysis). The DYNAFLOW laboratory is equipped with a Perkin Elmer Clarus 500 gas chromatograph with mass detector (GC/MS). The Clarus 500 is capable of collecting Total Ion Count (TIC) and Selected Ion Monitoring (SIR) ...
Manufactured by:Healgen Scientific, LLC. based inHouston, TEXAS (USA)
During testing, the whole blood, serum or plasma specimen reacts with the particle coated with anti-CK-MB antibodies. The mixture migrates upward on the membrane chromatographically by capillary action to react with capture reagent on the membrane and generate a colored line. The presence of this colored line in the test line region indicated a positive result, while its absence ...
Manufactured by:Shenzhen AIVD Biotechnology Co. , LTD. based inCheektowaga, NEW YORK (USA)
The FOB Rapid Test is a one-step chromatographic sandwich immunoassay. The test consists of: 1) a conjugate pad containing HB monoclonal antibody conjugated with colloidal gold. 2) a nitrocellulose membrane strip containing a test line (T line) and a control line (C line). The T line is pre-coated with another HB antibody, and the C line is pre-coated with a control line antibody ...