Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Clinical Specimen Equipment Supplied In Usa
24 equipment items found
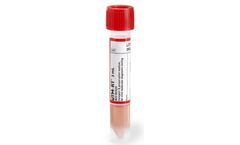
Manufactured by:MedSchenker based inEast Windsor, NEW JERSEY (USA)
For collection, transport, maintenance and long-term freeze storage of clinical specimens containing viruses, H1N1 and Adenovirus. It is comprised of premium components, 85% of which are sourced from within the ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended use: Genabio® Genital Ulcer Panel is a multiplex PCR assay for simultaneous detection of below mentioned three pathogens in clinical specimens. The three major causes of Genital Ulcer Disease (GUD) in the United States are Herpes simplex virus, Treponema pallidum (syphilis) and Haemophilus ducreyi (chancroid). ...
Manufactured by:Copan Diagnostics Inc. based inMurrieta, CALIFORNIA (USA)
UTM®: Universal Transport Medium™ for Collection, Transport, and Preservation of Clinical Specimens for Viral Molecular Diagnostic Testing ...
Manufactured by:ZeptoMetrix - ANTYLIA Scientific based inBuffalo, NEW YORK (USA)
They can be used for verification of assays, training of laboratory personnel and to monitor assay-kit lot performance. NATtrol products contain intact organisms and should be run in a manner similar to clinical ...
Manufactured by:ZeptoMetrix - ANTYLIA Scientific based inBuffalo, NEW YORK (USA)
They can be used for verification of assays, training of laboratory personnel and to monitor assay-kit lot performance. NATtrol products contain intact organisms and should be run in a manner similar to clinical ...
Manufactured by:ZeptoMetrix - ANTYLIA Scientific based inBuffalo, NEW YORK (USA)
It can also be used to determine a limit of detection (LOD), in diagnostic assay development, cross-reactivity studies or genomic sequencing. When used as a control for nucleic acid tests, the same protocols as those used to amplify extracted clinical specimens should be ...
Manufactured by:ZeptoMetrix - ANTYLIA Scientific based inBuffalo, NEW YORK (USA)
It can also be used to determine a limit of detection (LOD), in diagnostic assay development, cross-reactivity studies or genomic sequencing. When used as a control for nucleic acid tests, the same protocols as those used to amplify extracted clinical specimens should be ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
Intended use: Hardy Diagnostics Viral Transport Medium is recommended for the collection and transport of clinical specimens for the recovery of viral agents including, but not limited to, Herpes Simplex Type I, Herpes Simplex Type II, Cytomegalovirus (CMV), Influenza A, Influenza B, Respiratory Syncytial Virus (RSV), Echovirus, Adenovirus, etc. ...
Manufactured by:Biomed Diagnostics, Inc. based inWhite City, OREGON (USA)
Individually-sealed, sterile, flocked swabs with bear face shaped stopper and breakable shaft. For collecting SARS- CoV-2 and other clinical specimens with patient and physician comfort in ...
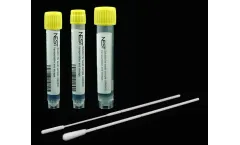
Manufactured by:GMP Plastic based inDover, DELAWARE (USA)
The Viral Transport Media (VTM) from Nest Scientific, offered by GMP Plastics, is engineered to facilitate the effective collection, transportation, and preservation of clinical specimens containing viruses, Chlamydia, Mycoplasma, and Ureaplasma. This VTM is encapsulated in a disposable sampler comprised of a 5 mL vial filled with 2.5 mL of transport media, ...
Manufactured by:Biomed Diagnostics, Inc. based inWhite City, OREGON (USA)
Biomed Diagnostics' VTM-C19 Transit Tube is intended for on-site collection and transport to the testing laboratory of human clinical specimens containing SARS-CoV-2 — the virus that causes COVID-19 disease in humans and/or (USA only) influenza A virus. Intended to be inoculated with specimens collected with a nasopharyngeal (NP) or ...
Manufactured by:Puritan Medical Products Co., LLC. based inGuilford, MAINE (USA)
2 ml Cary-Blair Transport Medium. 12 x 80 mm screw-cap polypropylene tube, orange cap. Intended for use in the preservation of clinical fecal and rectal specimens containing enteric bacteria from the collection site to the testing laboratory for examination and culture. Premium medical grade plastic components to improve product shelf life. For optimum ...
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
The Positivia COVID-19 Ag Rapid Test External Control Kit is intended for use with the OnSite COVID-19 Ag Rapid Test (REF ...
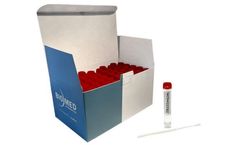
Manufactured by:Akuratemp based inArden, NORTH CAROLINA (USA)
Celsure Pre-Validated Specimen Transport Shippers are designed utilizing phase change technology for temperature control during specimen transportation between healthcare networks nationwide. These shippers feature a holdover time of 96 to 120 hours. They are tested to provide stable performance for Frozen, Refrigerated and Controlled Room Temperature applications. These shippers are integrated ...
Manufactured by:ZeptoMetrix - ANTYLIA Scientific based inBuffalo, NEW YORK (USA)
NATtrol products are ready to use, inactivated full process controls designed to evaluate performance of molecular tests. They can be used for verification of assays, training of laboratory personnel and to monitor assay-kit lot ...
Manufactured by:Anaerobe Systems based inMorgan Hill, CALIFORNIA (USA)
Brain Heart Infusion Broth (BHI BROTH) is an enriched non-selective media intended for the cultivation of most anaerobic bacteria and other fastidious ...
by:ProteoGenex, Inc. based inInglewood, CALIFORNIA (USA)
All fresh frozen tissue samples are collected under IRB approval by certified medical ...
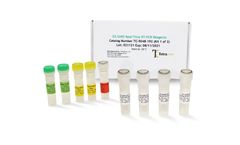
Manufactured by:Tetracore, Inc based inRockville, MARYLAND (USA)
EZ™-SARS-CoV-2 Real-Time RT-PCR is a real-time RT-PCR test intended for the qualitative detection of RNA from the SARS-CoV-2 in human nasal swab specimens from individuals suspected of COVID-19 by their healthcare provider. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, ...
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
The Aridia Dengue Serotyping Real-Time PCR Test is based on the real-time amplification and detection of dengue virus (DENV) serotypes 1-4 in a one-step ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. ...