Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Applications
Clinical Trial Support Equipment Supplied In Usa
83 equipment items found
Manufactured by:SafeHeal based inParis, FRANCE
Minimally invasive and fully reversible, Colovac is anchored by a vacuum seal above the anastomosis and can be removed during a routine endoscopic procedure. Clinical trials support Colovac’s effectiveness in improving and expediting patients’ recoveries from surgery. This is an especially important benefit for surgeons, clinicians, ...
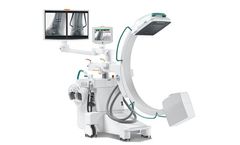
Manufactured by:Orthoscan, Inc. based inScottsdale, ARIZONA (USA)
With its all-in-one design, the Ziehm Solo FD is one of the most compact C-arms on the market – now equipped with CMOSline flat-panel technology to perform a broad portfolio of applications. Versatile viewing options and new dimensions in user friendliness offer maximum flexibility in the OR to support your clinical workflow. With the latest improvements in dose regulations, the Ziehm Solo ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
Post-exposure prophylaxis (PEP) regimens are designed to block or reduce impact of infections in individuals who have known or potential exposure to an infectious agent but are not symptomatic. Such regimens have been used successfully for other infections. In October 2018, SIGA entered into a Cooperative Research and Development Agreement (CRADA) with the United States Army Medical Research ...
Manufactured by:AMST-SMIT based inUniversity Park, ILLINOIS (USA)
The MAGNETOM Aera® Mobile MRI by Siemens Healthineers offers advanced medical imaging capabilities in a flexible, mobile format. This system is designed to provide high-resolution imaging and can be deployed in various settings, enhancing accessibility to MRI diagnostics. The mobile configuration includes standard and transportable options, with features optimized for ease of setup and use. ...
Manufactured by:Novum Medical Products based inAmherst, NEW YORK (USA)
Features: Supports 20 – 24 gallon bags, Glides easily with 2″ casters, Powder-coated steel lid and frame is attractive and easy to maintain, Non-slip foot pedal raises the blue, powder-coated steel lid. Specifications: Dimensions: 20.5”W x 16.73”D x ...
Manufactured by:MedCline based inSan Diego, CALIFORNIA (USA)
Reflux Relief System; 95% of MedCline users report better sleep. 1:1 access to a Sleep Specialist. CertiPUR-US®, gel-infused foam that’s built to last and for cooling comfort. Available in three sizes for optimal comfort. Patented arm pocket for flexible arm positioning. Includes the Reflux Relief Inclined Wedge, Insert Pillow, Therapeutic Body Pillow & set of removable, washable ...
Manufactured by:Catalyst MedTech based inPittsburgh, PENNSYLVANIA (USA)
A high-performance variable angle system with dual detectors that provide outstanding image quality for a full range of nuclear medicine ...
Manufactured by:NDS Surgical Imaging based inSarasota, CALIFORNIA (USA)
ZeroWire® G2 is a revolutionary third-generation advanced wireless HD-video system with the aim to transform technology into a clinical solution that supports the drive to improve patient outcomes, improve efficiency, and lower operating costs. Designed for user-friendly operation, ZeroWire G2 delivers full HD-video without noticeable video delay. Clinical teams can now enjoy greater mobility ...
Manufactured by:Clarus Medical, LLC based in55441, MINNESOTA (USA)
The Clarus Levitan Fiberoptic Stylet Has Been A Cornerstone Of The Difficult Airway Curriculum For Over A Decade. With years of strong clinical support and recent inclusion in the ASA Difficult Airway Algorithm, the Levitan is the trusted choice for thousands of doctors ...
Manufactured by:Integra LifeSciences based inPrinceton, NEW JERSEY (USA)
Providing the power, performance and protection of silver for your wound management ...
Manufactured by:RedHill Biopharma Ltd. based inTel-Aviv, ISRAEL
RHB-204 is a proprietary, fixed-dose oral capsule containing a combination of clarithromycin, rifabutin, and clofazimine, developed for the treatment of pulmonary NTM disease caused by Mycobacterium avium Complex (MAC). ...
by:Dominion Diagnostics based inNorth Kingstown, RHODE ISLAND (USA)
Dominion Diagnostics wants to help end the problem of prescription drug abuse among women. Our services support primary care physicians, gynecologists, and obstetricians that follow the latest industry recommendations to screen all reproductive-age women for signs of substance abuse. Dominion Diagnostics also offers other important clinical testing solutions to support women’s health. ...
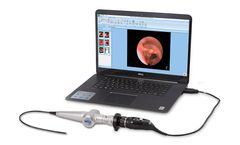
Manufactured by:Optim LLC based inSturbridge, MASSACHUSETTS (USA)
Optim is leading the way in providing portable swallowing diagnostics for the Speech-Language pathology ...
Manufactured by:Biopeptek Pharmaceuticals LLC based inMalvern, PENNSYLVANIA (USA)
Biopeptek is a leading manufacturer of synthetic peptide Active Pharmaceutical Ingredient (API). We offer custom APIs for pharmaceutical research & development organizations world-wide to support pre-clinical and clinical studies. We also support our clients for preparation of Chemistry, Manufacturing and Controls (CMC) regulatory ...
by:Vor Biopharma based inCambridge, MASSACHUSETTS (USA)
Licensed from the National Institutes of Health, VCAR33 is a CD33-directed chimeric antigen receptor T cell (CAR-T) therapy. A T cell therapy using the same CAR construct as VCAR33 is being studied in a multi-site Phase 1/2 clinical trial as an autologous monotherapy bridge-to-transplant for relapsed and/or refractory AML patients, sponsored by the National Marrow Donor Program ...
by:VisualDx based inRochester, NEW YORK (USA)
VisualDx is the award-winning diagnostic clinical decision support system used by more than 2,300 hospitals, clinics, and medical schools around the world. Get the answers you need when you need ...
Manufactured by:Catalyst MedTech based inPittsburgh, PENNSYLVANIA (USA)
A compact cardiac system with dual-detectors and a fixed 90° angle. It is capable of a wide variety of cardiac imaging procedures including static, dynamic, multi-gated and gated nuclear imaging ...
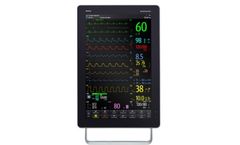
Manufactured by:Mindray North America based inMahwah, NEW JERSEY (USA)
Mindray believes the best way to predict the future is to create it. The revolutionary BeneVision N22/N19 is designed to optimize user experience by satisfying all your clinical demands. With visionary-stimulating design, benchmarking ease of use, confidence-maximizing innovations and workflow-transforming interoperability, BeneVision N22/N19 is creating tomorrow’s monitoring perspective ...
Manufactured by:Great Lakes NeuroTechnologies based inCleveland, OHIO (USA)
This data is processed in real-time via the Kinesia Web Portal to generate severity scores that correlate with clinician assessments every two minutes. The device supports continuous ambulatory monitoring, offering high sensitivity and reliability in measurements. Clinicians can access these reports and track compliance through a secure web platform that complies with regulatory ...
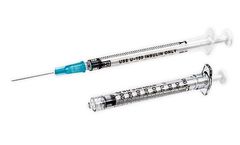
Manufactured by:Becton Dickinson and Company (BD) based inFranklin Lakes, NEW JERSEY (USA)
Our 1-mL BD Luer-Lok™ insulin syringe—available with either a detachable needle or a permanently attached needle—support many clinical uses and crucially prevent needle disengagement, medication leakage, and spray or tubing disconnect. We also offer a 1-mL insulin syringe with the BD Luer-Lok tip or the U-100 slip tip. These insulin syringes are all available at many drug ...