Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Collagen Matrix Equipment Supplied In Usa
21 equipment items found
Manufactured by:Fibralign Corporation based inUnion City, CALIFORNIA (USA)
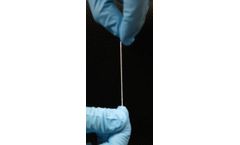
The BioBridge® Collagen Matrix is an FDA cleared surgical mesh designed for use in soft tissue support and repair. This thread-like device is made of medical-grade collagen, fabricated using Fibralign’s patented Nanoweave® technology. This novel product is the result of years of significant research collaboration with Stanford ...
by:Geistlich Pharma North America Inc. based inPrinceton, NEW YORK (USA)
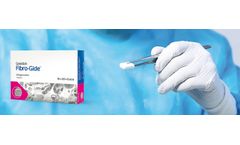
User Benefits: Geistlich Fibro-Gide® is a porcine, porous, resorbable and volume-stable collagen matrix, specifically designed for soft-tissue regeneration.1,4,10 The collagen matrix is made of reconstituted collagen and undergoes smart chemically cross-linking to improve its volume stability while ...
Manufactured by:Regenity based inParamus, NEW JERSEY (USA)
OssiMend Bioactive Moldable Bone Graft Matrix is a unique combination of mineral, bioactive glass, and collagen processed into strips and moldable pucks for bone grafting procedures. The products feature a perfect trio of mineral and bioactive glass particles, which are uniformly distributed throughout a 3-D collagen matrix. The ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
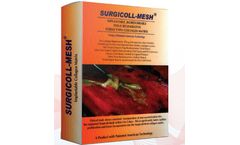
Surgicoll Mesh is a biocompatible collagen mesh that is flexible, strong yet soft. The collagen membrane is also supple when hydrated and handles like natural tissue. It readily conforms to the surgical site upon application and is easily sutured. Surgicoll- Mesh is constituted by Type-I collagen membrane that is free of contaminants like lipids, elastin and other immunogenic proteins. The ...
Manufactured by:Integra LifeSciences based inPrinceton, NEW JERSEY (USA)
SurgiMend MP is a 2mm thick acellular collagen matrix featuring a macroporous design that facilitates fluid drainage. SurgiMend MP provides the strength and support for complex hernia repair. Perforations in SurgiMend MP allow for tissue revascularization and integration, and play an important role in hernia ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
Tribio™ Backfill Plugs are a proprietary material delivered sterile and ready for use in any bony voids or gaps in the skeletal system. Consisting of hydroxyapatite, tricalcium phosphate and bioactive glass in a collagen matrix, the Backfill Plugs are sized to easily fit in surgically created osseous defects; 5.5mm dia. (for 6mm void) and 7.5mm dia. (for ...
Manufactured by:Spine Wave, Inc. based inShelton, CONNECTICUT (USA)
Tornado® Bioactive Bone Graft Matrix is a unique combination of mineral, bioactive glass, and collagen processed into strips and moldable pucks for bone grafting procedures. The product features a perfect trio of mineral and bioactive glass particles, which are uniformly distributed throughout a 3-D collagen matrix. The ...
Manufactured by:Misonix based inFarmingdale, NEW YORK (USA)
TheraGenesis Bilayer Wound Matrix is a collagen-based wound dressing that consists of two layers: a porcine tendon-derived atelocollagen sponge layer and a silicone film layer. It contains a non-adhesive gauze (TREX™) to reinforce the silicone film. The meshed type has slits to aid in the drainage of exudate. The biodegradable collagen ...
Manufactured by:Biorez Inc. based inNew Haven, CONNECTICUT (USA)
Unlike traditional implant materials that are either synthetic or biologic, BioBrace is a biocomposite of both. The proprietary biocomposite architecture features a highly porous type 1 collagen matrix (20 μm average pore size) reinforced with bioresorbable PLLA microfilaments (15 μm diameter), which are stylized green in the cross-section SEM image below. ...
Manufactured by:Regenity based inParamus, NEW JERSEY (USA)
OssiMend Bioactive Moldable Bone Graft Matrix is a unique combination of mineral, bioactive glass, and collagen processed into strips and moldable pucks for bone grafting procedures. The products feature a perfect trio of mineral and bioactive glass particles, which are uniformly distributed throughout a 3-D collagen matrix. The ...
Manufactured by:Parametrics Medical based inLeander, TEXAS (USA)
Synthetic bone void fillers are safe, biocompatible, and easy to use. These resorbable biomaterials are based on nano-crystalline hydroxyapatite (HA) and purified collagen technologies. These products are engineered to optimize the resorption rate. Our granules are composed of 60% HA and 40% β-Tricalcium Phosphate (TCP). The formulation provides optimal osteoconduction, and ...
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
We have taken a collagen plug and filled it with our OsteoGen ® Bone Graft resulting in an easy and affordable way to graft your extraction ...
Manufactured by:Regenity based inParamus, NEW JERSEY (USA)
A sponge-like onlay with a leak resistant top layer that provides effective protection against CSF leakage. When hydrated, this highly drapeable matrix is easily repositionable and conforms to the complex surfaces of the brain or spinal cord. The completely resorbable matrix does not adhere to surgical instruments or ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
BiFORM® Bioactive Moldable Matrix is a bioactive and osteoconductive bone graft that can be hydrated and used as a strip or molded into a putty to fill a bone ...
Manufactured by:Bio-Tissue, Inc. based inMiami, FLORIDA (USA)
TissueTech’s initial research and development efforts were focused on the development of its cornerstone cryopreservation technology – CRYOTEK. The CRYOTEK process is a proprietary tissue-processing method that preserves the innate biological and structural integrity of the natural placental tissue and therefore preserves and delivers the anti-inflammatory, anti-scarring, and ...
Manufactured by:GRAFIX PL, by Smith+Nephew, Inc. based inColumbia, MARYLAND (USA)
This product leverages human placental membranes, which are minimally manipulated to retain their native cells, growth factors, and extracellular matrix, ensuring the preservation of structural integrity. The membrane comprises three essential components of the placental structure, emphasizing the collagen-rich matrix crucial for wound healing. ...
Manufactured by:Nanova Biomaterials, Inc. based inColumbia, MISSOURI (USA)
Human bone is made up of plate-like hydroxyapatite (2-4 nanometer thick and up to 100 nanometer long) embedded in a collagen-rich protein matrix. Biocomposites’ containing hydroxyapatite (HA), benefit by improving biocompatibility or osteoconductivity, and reducing self-catalytic degradation. In literature implants with HA have been resorb and replaced by ...
Manufactured by:Sciton, Inc. based inPalo Alto, CALIFORNIA (USA)
Sciton’s Fractionated Erbium Laser Goes Deep Without the Downtime. Sciton’s fractionated erbium laser is the ideal treatment for scar revision, deep resurfacing, and healthy skin ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
Durepair Regeneration Matrix is a non-synthetic dura substitute used to repair defects in the ...
Manufactured by:LeMaitre Vascular, Inc. based inBurlington, MASSACHUSETTS (USA)
Artegraft is a bovine carotid artery graft. Its biological fibrous matrix is processed to enhance long-term patency and provide a tightly woven, cross-linked conduit that is flexible and compliant. For Functional Hemodialysis Access & Lower Extremity ...