Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Coronary Intervention Equipment Supplied In Usa
11 equipment items found
Manufactured by:MicroPort Scientific Corporation based inShanghai, CHINA
Extraordinary Deliverability. Ultra Low Profile. Target Eluting Technology of ...
Manufactured by:MicroPort Scientific Corporation based inShanghai, CHINA
CoCr Stent Platform. Reliable durable polymer-Styrene Butylenes Styrene. Uniform sine wave design provides uniform support strength and ...
Manufactured by:MicroPort Scientific Corporation based inShanghai, CHINA
TARGET All Comers Trial: 1,656 Patients from 20 sites in Europe. 1:1 RCT Real World open-label, non-inferiority trial Primary endpoint: TLF @12 months. Confirms that the Firehawk®, a low dose sirolimus eluting biodegradable polymer DES, is safe. and effective across a broad spectrum of patient and lesion complexity. ...
by:Supira Medical based inLos Gatos, CALIFORNIA (USA)
Developing a low-profile, high-flow percutaneous ventricular assist device (pVAD) for high risk coronary intervention and cardiogenic ...
Manufactured by:OrbusNeich Medical Company Ltd. based inShatin, N.T., CHINA
The Smart Solution for complex PCI Patients. Diamondback 360 Coronary Orbital Atherectomy System (OAS) reduces severe calcium, enabling successful stent delivery to help optimize stent expansion and percutaneous coronary intervention (PCI) ...
Manufactured by:QXMedical based inMontreal, QUEBEC (CANADA)
The Boosting Catheter is designed for use with guiding catheters and sheaths to assist delivery and exchange of interventional devices in coronary and peripheral vessels. The catheter offers exceptional trackability along with an atraumatic tip design and large internal passageways to facilitate complex procedures. The Boosting Catheter provides excellent support ...
Manufactured by:ACIST Medical Systems based inEden Prairie, MINNESOTA (USA)
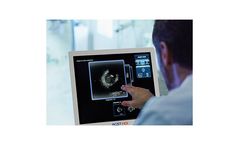
ACIST HDi High-Definition IVUS System enables optimized visualization to inform and illuminate coronary and peripheral intervention strategy through advanced imaging modes, improved deliverability of the Kodama IVUS Catheter and interactive compact console. ...
Manufactured by:Atrion Medical based inArab, ALABAMA (USA)
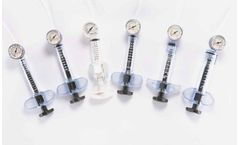
Atrion’s QL® Inflation Devices are perfect for a broad range of applications such as balloon catheterization, stent deployment and fluid delivery. ...
Manufactured by:Siemens Healthcare GmbH based inErlangen, GERMANY
The CorPath System is the first FDA-cleared and CE marked robotic platform designed for interventional physicians. During a CorPath Robotic-assisted intervention, physicians sit in a radiation-shielded workstation and use a set of joysticks and touchscreen controls that translate the physician’s movements into device control. Robotic-assisted intervention enables precise measurement of ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Delcasertib (KAI-9803) is a potent and selective δ-protein kinase C (δPKC) inhibitor. Delcasertib (KAI-9803) could ameliorate injury associated with ischemia and reperfusion in animal models of acute myocardial infarction ...
Manufactured by:Second Heart Assist, Inc. based inSalt Lake City, UTAH (USA)
Second Heart Assist objectives are to improve the quality of life by reducing the heart’s work-load and improve perfusion. The enhancement of renal function reduces risks associated with toxic contrasts used in percutaneous catheterization interventions (High Risk PCI). Additionally, the product helps patients recover from cardiogenic shock and get acute decompensating heart failure ...