Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Donor Graft Equipment Supplied In Usa
21 equipment items found
Manufactured by:Lohmann & Rauscher GmbH & Co. KG (L&R) based inRengsdorf, GERMANY
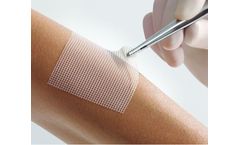
Ready-for-use mesh tulle saturated with white Vaseline and covered on both sides with glassine, for gentle treatment of superficial wounds. The wide-mesh tulle promotes exudate drainage.* The white Vaseline prevents adhesion to the wound.* This enables atraumatic dressing changes, ensures an undisturbed wound rest period and supports the natural course of healing.* The product can be cut to ...
Manufactured by:Cole Instruments Inc.. based inAlpharetta, GEORGIA (US) (USA)
Hair transplant procedures are sensitive and time-consuming and Cole Instruments reflects these facts. We design and manufacture instruments for donor harvesting, graft storage, recipient site preparation, graft preparation, tissue anesthesia and tumescence; hairline design, and sharps ...
Manufactured by:Humeca based inBorne the, NETHERLANDS
Every doctor will admit, burn wound treatment involves multiple challenges. There is a great risk of infections and poor epithelialization and the lack of auto graft donor sites is a limiting factor in achieving wound closure in cases of extensive skin ...
Manufactured by:Lohmann & Rauscher GmbH & Co. KG (L&R) based inRengsdorf, GERMANY
The high exudate absorption capacity of Suprasorb A promotes wound healing. The absorption of wound exudate causes the exchange of sodium ions with calcium ions, forming a gel. Bacteria and debris are trapped in the gel, creating a moist wound environment. Suprasorb A not only promotes wound healing but also offers a high degree of comfort due to its exceptionally soft structure and ease of ...
Manufactured by:Milliken & Company based inSpartanburg, SOUTH CAROLINA (USA)
AGILE dressings were developed to meet the unmet needs of patients and clinicians for a highly absorbent gellable dressing with increased structural integrity and conformability. AGILE dressings combine the patented Active Fluid Management® technology with an innovative weave of gelling fibrils. AGILE has the unique ability to pull excess moisture away from the wound bed, absorbs it into the ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
FORTIFY FLOWABLE Extracellular Matrix (ECM) is porcine small intestinal submucosa (SIS) and provides micronized ECM for surgical ...
Manufactured by:Lohmann & Rauscher GmbH & Co. KG (L&R) based inRengsdorf, GERMANY
Suprasorb A + Ag reduces the microbial load directly in the wound bed and is therefore especially suitable for wounds that are infected or at risk of infection - including MRSA or VRE*. In addition to having these properties, Suprasorb A + Ag is also a soft and supple wound covering that readily conforms to any wound bed. The high exudate absorption capacity of Suprasorb A + Ag comes to the fore ...
Manufactured by:Abena North America, Inc. based inMarina Del Rey, CALIFORNIA (USA)
Abena’s Foam Dressing is a hydrophilic polymer with a three-dimensional foam structure that is processed by the latest technological advancements to quickly absorb wound exudate and help maintain a moist wound healing environment. The dressing is designed to not stick to the wound and help minimize user’s discomfort upon removal. ...
Manufactured by:Abena North America, Inc. based inMarina Del Rey, CALIFORNIA (USA)
Abena’s Silicone Foam Dressing with Adhesive Border is a highly absorbent self-adherent silicone foam dressing consisting of a soft silicone contact layer, a flexible polyurethane foam pad, and a vapor-permeable, moisture-proof outer film with a low-profile border for added adhesion and flexibility. The dressing is comfortable as well as conformable. The triple-layered construction ...
Manufactured by:Abena North America, Inc. based inMarina Del Rey, CALIFORNIA (USA)
Abena’s Silicone Foam Dressing is a highly absorbent self-adherent silicone foam dressing consisting of a soft silicone contact layer, a flexible polyurethane foam pad, and a vapor-permeable, moisture-proof outer film. The silicone adhesive sticks to surrounding skin but not to the wound bed, helping to minimize pain and the risk of damage to the wound area. The dressing is comfortable, ...
Manufactured by:Lohmann & Rauscher GmbH & Co. KG (L&R) based inRengsdorf, GERMANY
The recommended dressing change interval is normally 2–4 days. Depending on the type of wound and its healing progress, the dressing can be left on the wound for a shorter or longer period (up to a maximum of 7 ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
BIAKO¯S™ ANTIMICROBIAL SKIN & WOUND CLEANSER works synergistically to cleanse and remove microbes from the wound bed to help eliminate planktonic, immature and mature ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
BIAKO¯S™ ANTIMICROBIAL SKIN & WOUND IRRIGATION SOLUTION works synergistically to irrigate and remove microbes from the wound bed to help eliminate planktonic, immature and mature ...
Manufactured by:Misonix based inFarmingdale, NEW YORK (USA)
TheraGenesis Bilayer Wound Matrix is a collagen-based wound dressing that consists of two layers: a porcine tendon-derived atelocollagen sponge layer and a silicone film layer. It contains a non-adhesive gauze (TREX™) to reinforce the silicone film. The meshed type has slits to aid in the drainage of exudate. The biodegradable collagen matrix provides a scaffold for cellular invasion and ...
Manufactured by:Aroa Biosurgery Limited based inMangere, NEW ZEALAND
Myriad Morcells is a morcellized (powdered) format of Myriad Matrix for soft tissue repair and complex wounds. Myriad Morcells delivers a bolus of biologically important extracellular matrix (ECM) proteins that help kick start ...
Manufactured by:Lohmann & Rauscher GmbH & Co. KG (L&R) based inRengsdorf, GERMANY
Vliwasorb Pro is a superabsorbent dressing for wounds with moderate to very high levels of exudate. The very high absorption and retention capacity of Vliwasorb Pro protects wound edges from maceration. Vliwasorb Pro promotes wound healing by maintaining a moist wound environment. The development of odour during the treatment is decreased. Proteases (e.g. MMPs, elastase) are reduced.* Vliwasorb ...
Manufactured by:Kerecis based inArlington, VIRGINIA (USA)
Kerecis Omega3 OR is not available in all countries and may be known by different names than used on this web-page. Products, product names, indications and claims vary between countries. Not all indications and claims below are for example cleared by the US ...
Manufactured by:Aroa Biosurgery Limited based inMangere, NEW ZEALAND
Endoform Antimicrobial Restorative Bioscaffold and Endoform Natural Restorative Bioscaffold are unique extracellular matrix (ECM) products for the management of acute and chronic wounds. Endoform products support all phases of wound healing and are appropriate for use early in wound management to restore protease balance and advance healing to the proliferative ...
Manufactured by:Aroa Biosurgery Limited based inMangere, NEW ZEALAND
Myriad Matrix is an engineered extracellular matrix (ECM) for soft tissue repair, reinforcement and complex wounds. Myriad Matrix has been specifically designed to help maximize tissue repair while providing versatility and adaptability across a wide range of surgical applications. Myriad Matrix is designed to be easily customizable for a wide range of anatomical sites and surgical needs and can ...
Manufactured by:Kerecis based inArlington, VIRGINIA (USA)
An FDA 510(k) medical device indicated for managing wounds. Not subject to the May 31, 2021, FDA 351 regulation. Kerecis Omega3 MicroGraft is an intact fish-skin graft that has been fragmented into smaller pieces to mold and insert into deep wound spaces and irregular ...