Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
End Stage Renal Disease Equipment Supplied In Usa
20 equipment items found
by:Alucent Biomedical Inc. based inSalt Lake City,, UTAH (USA)
Dialysis is a life-saving treatment for patients with end-stage renal disease. Creation of arteriovenous (AV) fistulas are often the best option for permanent vascular access in these patients. Once mature, AV fistulas allow a conduit for regular dialysis, however, failure rates remain high (reaching up to 70%) due to lack of ...
Manufactured by:NxStage Medical, Inc. based inLawrence, MASSACHUSETTS (USA)
NxStage System One is a simple, flexible and portable system providing the growing number of people with end-stage renal disease better options for hemodialysis treatments. NxStage System One was designed with patients in mind to provide simplicity, flexibility and portability without compromising safety. System One was designed ...
Manufactured by:Baxter International Inc. based inDeerfield, ILLINOIS (USA)
HDx enabled by THERANOVA is a new dialysis therapy we are pioneering for patients with end-stage renal disease. The THERANOVA dialyzer features an innovative membrane, which provides an expanded hemodialysis therapy ...
Manufactured by:Baxter International Inc. based inDeerfield, ILLINOIS (USA)
Amia automated peritoneal dialysis (APD) with the Sharesource remote patient management platform is the first and only APD system to include user-friendly features that help guide end-stage renal disease patients through home peritoneal dialysis (PD) therapy, while keeping them remotely connected with their healthcare ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Ellipsys® Vascular Access System provides a groundbreaking, minimally invasive and cost effective method to create a percutaneous arteriovenous (AV) fistula for hemodialysis access in patients with End-Stage Renal ...
Manufactured by:Cabaletta Bio, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
PLA2R-CAART is being developed to treat patients with PLA2R-associated membranous nephropathy (MN), a B cell-mediated autoimmune disease that affects the kidneys. B cells produce autoantibodies, which are believed to form immune complexes that are deposited at the glomerular basement membrane, causing damage of the filtration barrier, and leading to proteinuria. Over ...
Manufactured by:OPKO Health, Inc. based inMiami, FLORIDA (USA)
OPK88004 is being assessed as a treatment for muscle wasting, decreased physical strength and function, sarcopenia, and frailty in end stage renal disease (ESRD) patients on dialysis and for prostate cancer patients on Androgen Deprivation Therapy (ADT). The selective anabolic effects of SARM on lean body mass and physical ...
Manufactured by:Alkahest, Inc. based inSan Carlos, CALIFORNIA (USA)
Another important detrimental chronokine to the biological process of aging is known as beta-2 microglobulin ...
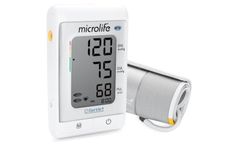
Manufactured by:Microlife Corporation based inWidnau, SWITZERLAND
The A6 PC is equipped with AFIB technology - the world’s number one atrial fibrillation (AF) detection system while taking blood pressure measurements at home. The blood pressure data can be uploaded via USB (cable included) to a computer, which helps to easily track, analyse and understand the health condition by using our free BPA software. Moreover, this device was honoured by the ...
Manufactured by:Microlife Corporation based inWidnau, SWITZERLAND
The A200 AFIB is equipped with Microlife’s unique AFIB technology, which makes it possible to detect atrial fibrillation while measuring blood pressure at home. Two out of three atrial fibrillation related strokes can be prevented if they were diagnosed early and treated accordingly. Additionally, the PC link function of this device allows the user to analyse and track health condition by ...
Manufactured by:Microlife Corporation based inWidnau, SWITZERLAND
The A200 AFIB is equipped with Microlife’s unique AFIB technology, which makes it possible to detect atrial fibrillation while measuring blood pressure at home. Two out of three atrial fibrillation related strokes can be prevented if they were diagnosed early and treated accordingly. Additionally, the PC link function of this device allows the user to analyse and track health condition by ...
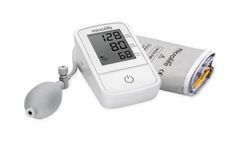
Manufactured by:Microlife Corporation based inWidnau, SWITZERLAND
N2 Easy of Microlife is a semi-automatic blood pressure monitor and allows high accuracy. It only requires two batteries, which enables long term use. With its one-button operation, this device is very easy to use and simultaneously automatically detects for irregular heartbeat during each measurement. ...
Manufactured by:Amicus Therapeutics, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
Galafold® is indicated for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data. This indication is approved under accelerated approval based on reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate. Continued approval for this indication may be contingent ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Miromatrix is developing bioengineered organs for transplantation, and our technology can be applied across the spectrum of donor needs. We are currently focused on bioengineering transplantable kidneys (MiroKidney) and livers (MiroLiver), but we believe our technology can be used to engineer other in-demand organs, such as lungs, pancreases, hearts and other internal organs or vascularized ...
Manufactured by:Pfizer Inc. based in, NEW YORK (USA)
ACCUPRIL® is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat. Quinapril hydrochloride is chemically described as [3S-[2[R*(R*)], 3R*]]-2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid, monohydrochloride. Its empirical formula is C25H30N2O5 ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-300-IV is being developed for the treatment of diabetic complications, including diabetic kidney disease known as diabetic ...
by:Solinas Medical, Inc. based inSanta Clara, CALIFORNIA (USA)
510(k) cleared device for cardiovascular patching where repeated access is desired. Designed to reduce leakage after cannulation in AV access systems. Constructed of novel composite material. Available in multiple sizes & shapes that conform to vessels. Preclinical testing demonstrates enhanced durability and resistance to ...
Manufactured by:Pfizer Inc. based in, NEW YORK (USA)
ACCURETXC is a fixed-combination tablet that combines an angiotensin-converting enzyme (ACE) inhibitor, quinapril hydrochloride, and a thiazide diuretic, ...
by:Endomimetics based inBirmingham, ALASKA (USA)
In many medical settings, devices are commonly used to help doctors treat patients. For example, stents are often used to open blocked arteries, repair torn vessels, and even to keep nasal passages from swelling shut after surgery. Unfortunately these devices fail often resulting in additional surgeries and often replacements of the original ...
Manufactured by:Vascudyne, Inc. based inStillwater, MINNESOTA (USA)
Synthetic vascular grafts currently used in clinic have poor long term outcomes and low patency rates. Hyperplasia, calcification, foreign body immune response, infection, and atheroma formation are commonly encountered limiting the use of synthetic grafts, with none available for small diameter applications like coronary ...