Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Femoral Artery Equipment Supplied In Usa
41 equipment items found
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
Our Neuro System Trainer extends from the femoral arteries to the pericallosals and M2, and A2 segments, and features six aneurysms of varying sizes. The left and right vertebrals are customized to simulate both a young and mature ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
Our Neuro System Trainer extends from the femoral arteries to the pericallosals and A2, M2 segments, and features six aneurysms of varying sizes. The left and right vertebrals are customized to simulate both a young and mature adult. ...
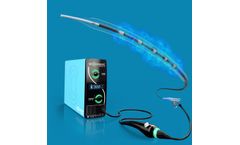
Manufactured by:Viant Medical based inFoxborough, MASSACHUSETTS (USA)
Complex interventional devices. Steerable, balloon, and RF ablation catheters. Closure devices (femoral arterial and venous access). Introducers for transradial artery access. ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
Our Neuro System Trainer extends from the femoral arteries to the pericallosals and A2, M2 segments, and features six aneurysms of varying sizes. The left and right vertebrals are customized to simulate both a young and mature adult. To secure your vessel during testing or demonstrations, we offer 3D/2D Stabilizing Platforms. ...
Manufactured by:Prytime Medical Devices, Inc. based inBoerne, TEXAS (USA)
The REBOA Convenience Set is compatible with all Prytime Medical™ REBOA catheters and provides the clinician with the components needed for common femoral artery access, REBOA placement, and external fixation of the ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
The Transcatheter Aortic Valve Replacement model is our transparent, versatile platform for training and product development. This vessel features two replaceable aortic valves, the left ventricle, and a full aorta down to the femoral arteries. Femoral access and modular heart valves allow for full simulation of transcatheter therapy scenarios ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
The EXOSEAL Vascular Closure Device is designed for a safe, simple, and secure close. The EXOSEAL VCD is indicated for femoral artery puncture site closure, reducing times to hemostasis and ambulation in patients who have undergone diagnostic or interventional catheterization procedures using a standard 5F, 6F, or 7F vascular sheath introducer with up to a 12-cm ...
Manufactured by:Insightra Medical Inc, a Mundomedis Group Company based inClarksville, TENNESSEE (USA)
This ULTRA 7FR IAB catheter is a true 7Fr (including the wrapped balloon), so it will pass through all 7Fr sheaths. 7Fr means a 23% reduction on cross-sectional area (vs. a competitive 8Fr), which results in over 20% better distal blood flow in a 4.0mm femoral artery. ...
Manufactured by:Red Vascular Technology, LLC based inTampa, FLORIDA (USA)
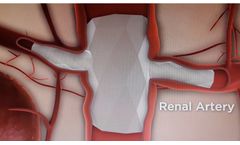
Red Vascular’s non-modular, branched endoprosthesis represents an ideal solution for aortic aneurysmal disease with branch artery involvement. Its design and delivery through a single femoral artery site are superior to modular, fenestrated grafts currently used to treat complex aneurysms. Fenestration adds several complex ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Niguldipine free base exhibits dose-dependent and sustained increases in coronary blood flow. Niguldipine free base also increases perfusion in the kidneys and femoral arteries, but the effect is temporary and to a lesser extent. The effect of Niguldipine free base on myocardial metabolism is not ...
Manufactured by:Medinol based inTel Aviv, ISRAEL
ChampioNIR is a hybrid stent combining Nitinol alloy structure with an elastomer micro-fiber mesh. Fracture resistance during contraction, compression, torsion, extension and flexion. Outstanding radial support and conformability to the vessel natural form and motion. Unique delivery system for accurate stent deployment. Superior flexibility for better deliverability. ChampioNIR is the first ...
Manufactured by:Shockwave Medical Inc. based inSanta Clara, CALIFORNIA (USA)
Quicker Cycle Time With 2x Faster Pulsing. 2X Faster Pulsing: Shockwave M5+ delivers the same amount of energy quicker with the same safety and efficacy profile as Shockwave ...
Manufactured by:Viant Medical based inFoxborough, MASSACHUSETTS (USA)
Heart positioners, Aortic punches, Syringes, Arterial, femoral, and venous cannulae for extracorporeal circuits, Heart valve holders, Delivery ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
AN EXCELLENT ALTERNATIVE FOR REVASCULAZATION OF SFA AND BTK ARTERIES.MagicTouch PTA is intended to prevent re-narrowing of superficial femoral and popliteal arteries in patients with peripheral artery ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
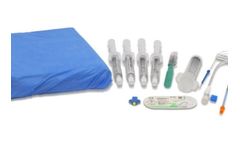
Practice both Femoral Vascular Access as well as Femoral Regional Anesthesia with Simulab's Regional Anesthesia and Vascular Access Femoral Training Package. The package includes everything you need to build on your practice. Start your training on the Venipuncture Pad with Nerves, move to femoral access with the ultrasound compatible FemoraLineMan tissue, and move up to the regional anesthesia ...
Manufactured by:Delta Med based inViadana, ITALY
The arterial catheters of the DELTA ARTERIAL CANNULA line guarantee a safe and uncomplicated positioning and atraumatic punctures of the peripheral ...
Manufactured by:SIFSOF LLC based inLos Angeles, CALIFORNIA (USA)
The color Doppler USB and Wireless 3 in 1 Ultrasound Scanner: SIFULTRAS-3.33 includes Linear, convex and cardiac scan modes all in one probe. It is compatible with Windows, Android, and IOS. In addition, there is no need to change the probe’s head in which this can easily done by changing the ...
Manufactured by:SONOSIF - WiFi Ultrasound Scanners based inLos Angeles, CALIFORNIA (USA)
The 3 in 1 Color Doppler USB and portable ultrasound Scanner: 3in1-CLC4CD is a Portable ultrasound machine that includes Linear, convex, and cardiac scan modes all in one probe. It is compatible with Windows, Android, and IOS. In addition, there is no need to change the probe’s head in which can easily be done by changing the ...
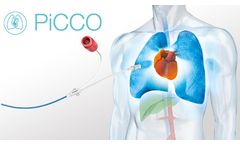
Manufactured by:Getinge AB based inGöteborg, SWEDEN
Cost-efficient tool for hemodynamic monitoring at the highest level. Monitoring physiological parameters is essential for the goal directed management of critical care patients. ...
Manufactured by:Simulab Corporation based inSeattle, WASHINGTON (USA)
Simulab's FemoraLineMan System is an ultrasound-compatible femoral line vascular access trainer. The product offers an effective training solution for central venous or arterial access at the femoral site. ...