Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Heart Procedure Equipment Supplied In Usa
7 equipment items found
Manufactured by:Zimmer Biomet CMF and Thoracic based inJacksonville, NEW JERSEY (USA)
SternaLock Blu is specifically designed by cardiothoracic surgeons for primary sternal closure following median sternotomies for open-heart procedures. Zimmer Biomet has nearly two decades of clinical experience. Explore the legacy studies and whitepapers of our primary closure ...
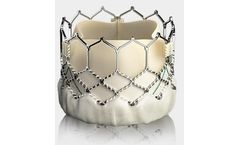
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Builds upon the proven strengths of the SAPIEN valve with 5 years of durability. The SAPIEN 3 valve is the only valve approved for valve-in-valve procedures in both the aortic and mitral positions, allowing patients at high or greater surgical risk to avoid an additional open heart ...
Manufactured by:Zimmer Biomet CMF and Thoracic based inJacksonville, NEW JERSEY (USA)
An innovative, sternal closure system designed to approximate, compress and rigidly fixate the sternum following open-heart surgery in patients with poor bone ...
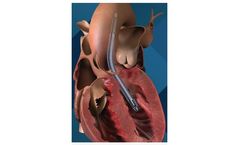
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
The Impella 5.5 with SmartAssist heart pump delivers full cardiac support, allowing the heart to rest and enabling the heart to achieve its natural pumping function without additional support. This heart pump is designed for long-duration support, enables patient mobility and optimizes recovery by using real-time ...
Manufactured by:Danlee Medical Products, Inc. based inSyracuse, NEW YORK (USA)
The Reusable Holter Monitor Carrying Case - item #CA155-EM is made for use with the Burdick 4250 Holter Recorder, the Forest Medical Model 5900 Patient Recorder and the Braemar DL900 Series Holter Monitors. This product is made from lightweight, washable Nylon that is both water and abrasive resistant and also sports a thermal dot to mark the event button. A shoulder strap and/or belt may be ...
Manufactured by:Abbott based inSanta Clara, ALABAMA (USA)
The TriClip™ Transcatheter Tricuspid Valve Repair System, developed by Abbott, is specifically designed for the minimally invasive treatment of severe tricuspid regurgitation (TR), particularly in patients who face high surgical risks. This device provides a transcatheter edge-to-edge repair (TEER) approach, leveraging proven leaflet repair technology to achieve consistent reduction in TR. ...
Manufactured by:U.S. Stem Cell, Inc. (USRM) based inSunrise, FLORIDA (USA)
Stem Cell’s lead product candidate is MyoCell®, a muscle stem cell therapy that is intended to improve cardiac function months or even years after a patient has suffered severe heart damage due to a heart attack. The procedure involves a physician removing a small amount of muscle from the patient’s thigh. From this muscle ...