Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
In Vitro Study Equipment Supplied In Usa
70 equipment items found
Manufactured by:Cabaletta Bio, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
Precision Approach to Address the Underlying Cause of Disease: Our second candidate, MuSK-CAART, is designed to treat myasthenia gravis (MG), an autoimmune disease affecting the neuromuscular junction that can lead to motor impairment, muscle weakness, and respiratory ...
by:Aphios Corporation based inWoburn, MASSACHUSETTS (USA)
A combination therapy consisting of PKC agonists with or without HDAC inhibitors, designed to reactivate latent HIV reservoirs so that HIV can be eliminated by antiviral therapy and eradicated from the body. HIV Latency: Although highly active antiretroviral therapy (HAART) is undoubtedly a lifesaving therapy for millions of AIDS patients, the persistence of latent HIV-infected cellular ...
Manufactured by:Prellis Biologics, Inc. based inSan Francisco, CALIFORNIA (USA)
Large organoids and tissue models can be grown in vitro in flow-free culture, reducing the time to organoid development while maintaining the properties of a 3D organoid. Organoid scaffolds are laser printed using transplantable hydrogel material allowing for both in vitro studies and transplantation of the same tissues into animal models. ...
Manufactured by:BiomX based inNess Ziona, ISRAEL
This program leverages know-how and capabilities acquired under our previous programs with regard to topical formulation and clinical testing. In preclinical in vitro studies, BX005 was shown to be active against over 90% of strains of S. aureus isolated from the skin of subjects from U.S. and Europe, including antibiotic resistant strains. ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Amlodipine hydrochloride is a biologically active drug used to lower blood pressure and prevent chest pain. Amlodipine hydrochloride has shown synergistic effects with antimicrobial drugs in in vitro studies, especially against carbene peptide-resistant Acinetobacter baumannii. Amlodipine hydrochloride can be used in combination with other antibiotics to enhance ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Cytokine production induced by PHA mimics that seen after brisk stimulation of human immune cells by an immunogenic pathogen or an infection. We obtain source material PBMCs from FDA-licensed blood banks. Preclinical data from animal and in vitro studies, as well as biologic data from patients in clinical trials, demonstrate that IRX-2 acts in multiple ways to ...
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
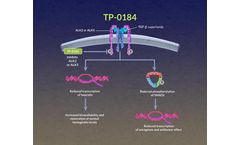
TP-0184 is an investigational inhibitor of activin receptor-like kinase 2 (ALK2) and ALK5 (also known as TGFβR1). This bimodal inhibitor is believed to downregulate multiple TGF-β superfamily signaling pathways and has shown antitumor activity in several ...
Manufactured by:CELLINK based inBoston, MASSACHUSETTS (USA)
PCL provides a reinforcing structure to load-bearing tissue constructs, including bone grafts and osteochondral plugs developed for in vitro and in vivo studies. It can also provide a temporary scaffold for overhanging ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
One of the primary functions of a bone graft material is to provide an osteoconductive surface for new bone formation. Bone regeneration is a cellular process that involves the interaction of osteoblasts and stem cells with the surface of a bone graft material. Studies have shown that the bone graft surface structure and morphology can impact how cells interact with a material. The TrelCor ...
Manufactured by:Regenity based inParamus, NEW JERSEY (USA)
A sponge-like onlay with a leak resistant top layer that provides effective protection against CSF leakage. When hydrated, this highly drapeable matrix is easily repositionable and conforms to the complex surfaces of the brain or spinal cord. The completely resorbable matrix does not adhere to surgical instruments or ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical offers the only meniscal allograft with both bone and meniscus sterilized through the BioCleanse® Tissue Sterilization Process. The BioCleanse processed meniscus graft provides a sterilized alternative to traditional aseptically processed meniscus allografts and sets the standard for safety and ...
Manufactured by:Centerline Biomedical based inCleveland, OHIO (USA)
Centerline Biomedical’s Intra-Operative Positioning System (IOPS) technology is an innovative, 3D GPS-like surgical navigation technology that improves endovascular procedure outcomes and reduces radiation exposure. ...
Manufactured by:Regenerative Medical Solutions Inc. (RMS) based inMadison, WISCONSIN (USA)
ILC Cells kit contains islet like cells and supplement media. This product has been specially formulated to contain cryopreserved single cells that are frozen in vials. The islet like cells in this kit are derived from human iPSC and closely resemble human cadaveric islets from a morphological and functional ...
Manufactured by:Creative Bioarray based inShirley, NEW YORK (USA)
Smooth muscle is responsible for the contractility of hollow organs, such as blood vessels, the gastrointestinal tract, the bladder, and the uterus. Its structure differs greatly from that of skeletal muscle. The human esophagus contains three layers of muscle in its walls, the outer longitudinal and inner circular layers of the main muscular coat and the muscular layer of the mucosa. Visceral ...
Manufactured by:Garwood Medical Devices, LLC based inBuffalo, NEW YORK (USA)
Currently under investigation to study the elimination of biofilm infections on prosthetic knee implants during early intervention procedures while maintaining the current standard of ...
Manufactured by:Imbed Biosciences based inMiddleton, WISCONSIN (USA)
Chronic, nonhealing wounds of varying types are treated with Microlyte® Matrix. Learn more about its use and effectiveness. Engineered to naturally contour to the underlying wound ...
Manufactured by:BiomX based inNess Ziona, ISRAEL
We are developing BX004, a phage therapy for CF patients with chronic Pseudomonas aeruginosa (P. aeruginosa) respiratory infections, a main contributor to morbidity and mortality in this disease. Phase 1b/2a clinical study in CF patients is composed of two parts. Results from Part 1 aimed to evaluate the safety, pharmacokinetics, and microbiologic/clinical activity are expected in Q3 of 2022. ...
Manufactured by:CONMED Corporation based inLargo, FLORIDA (USA)
With it's small size and innovative instrumentation, the Y-Knot® Flex System was designed to help achieve optimal anchor placement. The anchors provide up to 55% greater fixation strength than conventional 3.0mm press fit anchors while removing up to 80% less bone.1 The curved and percutaneous delivery systems enable delivery around a curve and perpendicular placement for posterior SLAP and ...
Manufactured by:Aedicell, Inc. based inRio Grande, NEW JERSEY (USA)
The only product on the market that combines human tissue sourced from the amniotic/chorionic membrane, umbilical cord AND placental disc —Dermavest®. This combination delivers a myriad of cell attachment proteins (CAP) including collagen, proteoglycans, polysaccharides and cytokine/growth factors (GF’s) that, combined with the structural aspects of the placental connective tissue ...
Manufactured by:Attwill Medical Solutions based inLodi, WISCONSIN (USA)
FILTERGuard™ is a disposable IV filter with CHG. The sterile, single-use disposable IV filter is infused with chlorhexidine gluconate (CHG) to help reduce the incidence of bloodstream infections, local infections and skin colonization in patients with IV catheters. This innovative product is designed as an in-line accessory for catheters to trap and kill microbial contaminants from catheter ...