Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Indwelling Catheter Equipment Supplied In Usa
11 equipment items found
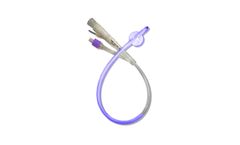
Manufactured by:Light Line Medical, Inc. based inSalt Lake City, UTAH (USA)
A catheter-associated urinary tract infection (CAUTI) is one of the most common infections a person can contract in the hospital or nursing home, according to the American Association of Critical-Care Nurses (AACCA, 2012). Indwelling catheters are the typical cause of this ...
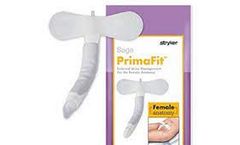
Manufactured by:Sage Products LLC based inCary, ILLINOIS (USA)
The innovative female external urine management system acts as a transition from indwelling catheters to independent continence. It is designed to address the factors required to effectively manage urinary incontinence – fit, securement, and ...
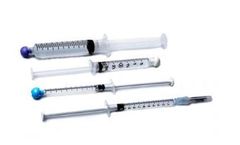
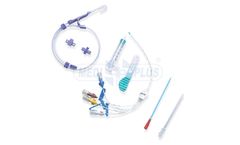
Manufactured by:SAI Infusion Technologies based inLake VIlla, ILLINOIS (USA)
Use prefilled syringes for flushing, locking or sampling through an indwelling catheter or port system. This prefilled option can save you time, space and money. All syringes are manufactured under controlled conditions using USP grade ingredients. Other options include Isotonic NaCL syringes and Catheter Maintenance Kits. ...
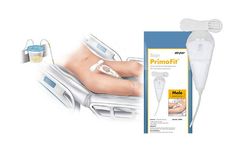
Manufactured by:Sage Products LLC based inCary, ILLINOIS (USA)
This first-of-its-kind male urine management system uses continuous suction to help promote early catheter removal, addressing the #1 risk factor of catheter-associated urinary tract infections (CAUTI).1 PrimoFit serves as an alternative to external collection devices or, when appropriate, indwelling catheters, while ...
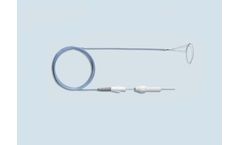
Manufactured by:PFM Medical, Inc. based inCarlsbad, CALIFORNIA (USA)
The Multi-Snare Device is a patented, highly efficient multiple loop retrieval snare with a dual-plane design to grasp objects from numerous angles. Designed with various reliable pull-force strengths, the kink resistant nitinol dual loop system grants the ability to grasp, manipulate or assist from any angle or vessel sidewall. The Multi-Snare MAX Device has been designed with the same ...
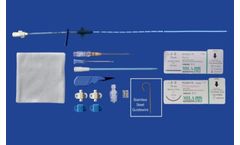
Manufactured by:MILA International, Inc. based inFlorence, KENTUCKY (USA)
Indicated to provide central venous access for delivery of fluids and medications and blood sampling. Other uses include measuring central venous pressure and administration of hypertonic solutions (such as TPN). Contraindicated in coagulopathic patients. Always use a dedicated lumen for blood sampling if ...
Manufactured by:Shree Umiya Surgical Pvt. Ltd. based inAhmedabad, INDIA
Central Venous Catheter made of specially formulated and biocompatible Polyurethane material provides strength during insertion and also softens at body temperature to conform to the body tissues and reduces the risk and vascular trauma. ...
Manufactured by:dBMEDx based inLittleton, COLORADO (USA)
Designed specifically for busy healthcare teams adhering to best demonstrated practices for CAUTI (catheter acquired urinary tract infection) ...
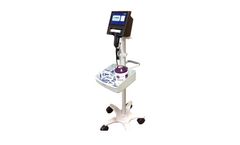
Manufactured by:NanoVibronix based inElmsford, NEW YORK (USA)
UroShield is a urology therapy device that is composed of two components: Disposable Clip – Intended for single use and to be discarded with catheter replacement. Driver – A portable unit (AC or battery powered) that provides power to the disposable clip. The ultrasonic waves generated by the clip create an acoustic shield on the surfaces of the catheter to interfere with the ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Remodulin is a prostacyclin vasodilator indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts (23%), or PAH ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
MARK-IN-2 is a potent microtubule affinity regulating kinase (MARK) inhibitor with an IC50 of 5 ...