Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Infection Risk Equipment Supplied In Usa
88 equipment items found
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
Chosen more often than any other to provide an effective barrier against bacterial transfer and standardize clinical protocols for all patients and care areas. Help enhance safety and efficiency by choosing industry-leading infection control technology. When you choose ICU Medical needlefree IV sets and connectors with patented Clave™ technology, you get an effective ...
Manufactured by:Cepheid based inSunnyvale, CALIFORNIA (USA)
Rapid and accurate detection of colonization facilitates targeted infection control practices: Optimize pre-admission workflow and counseling; Enables measures to reduce endogenous infection risk, including decolonization; Supports measures to reduced exogenous infection risk, including barrier/contact ...
Manufactured by:Quest Medical Inc. based inAllen, TEXAS (USA)
Quest’s manifold sets focus on safe and accurate infusion therapy delivery with minimal priming volumes and high flow rates. Our newest manifold, the ExoFlex, was designed to assist with reducing infection risks by developing a manifold that could be rigid or flexible in seconds – preventing a break in the line in between departments. Our full product ...
Manufactured by:Sage Products LLC based inCary, ILLINOIS (USA)
Our early prepping systems help address infection risk factors on three main reservoirs of bacteria: the nares, the oral cavity, and the skin. This can help standardize your pre-op approach for maximum efficiency and enhanced compliance to ...
Manufactured by:Enthermics Medical Systems based inMenomonee Falls, WISCONSIN (USA)
Patient warming is so much more than providing comfort during an uncertain medical crisis – keeping patients warm aids in the recovery process and reduces surgical site infection ...
Manufactured by:Enthermics Medical Systems based inMenomonee Falls, WISCONSIN (USA)
Patient warming helps reduce surgical site infections and aids in the recovery process. Use the affordable, durable countertop EC350 blanket warmers in the operating room or labor and delivery rooms to keep patients warm throughout the entire procedure, reducing surgical site infection risks. ...
Manufactured by:Sage Products LLC based inCary, ILLINOIS (USA)
Using a basin to clean your patients may increase the risk of infection. Studies have shown basins are often contaminated with bacteria, including multi-drug resistant organisms and gram-negative bacilli.1,2 Our rinse-free barrier cream cloths are the #1 hospital brand3 and provide the ideal barrier for all clinical ...
Manufactured by:Advin Health Care based inAhmedabad, INDIA
Plastibell is a single-use disposable plastic device mainly used to circumcise infants, but it can be used for boys up to 12 years of age. The Plastibell plastic ring is placed under the foreskin and secured with a circumferential ligature, which prevents bleeding when the distal foreskin is ...
Manufactured by:Xenco Medical based inSan Diego, CALIFORNIA (USA)
The SETx Lumbar Interbody System is entirely disposable, eliminating the need for the processing and sterilization of instrument trays, which is not only expensive and cumbersome but increases the risk of infection, breakage and misplacement. The SETx Lumbar Interbody System features instruments that are always in peak condition, always calibrated, and pre-loaded ...
Manufactured by:Xenco Medical based inSan Diego, CALIFORNIA (USA)
Sterile-packaged, each CancelleX implant is the first titanium foam implant of its kind to come pre-attached to a disposable, composite polymer delivery instrument, eliminating the need for the processing and sterilization of instrument trays, which is not only expensive and cumbersome but increases the risk of infection, breakage and misplacement. ...
Manufactured by:Anika Therapeutics, Inc. based inBedford, MASSACHUSETTS (USA)
Anika’s ToeMate® System is a strong, easy to install intramedullary bone screw implant with proven taper lock fixation intended for the correction of a hammertoe deformity. The implant components are completely embedded in the phalangeal bone, preventing implant exposure and complications associated with post-op pin tract infection. The taper lock provides a press fit connection between ...
Manufactured by:Accelerate Diagnostics, Inc. based inTucson, ARIZONA (USA)
Easier for the lab. Earlier for clinicians. Fast MIC results. Direct from positive blood cultures. Now with optional ID, and affordably ...
Manufactured by:CrossBay Medical based inSan Diego, CALIFORNIA (USA)
We are committed to improving women’s health through our innovative technology platform and suite of patient-friendly concepts. We want to deliver a more positive and gentle experience during uterine access procedures. We understand that changing the status quo may be a little uncomfortable at first. But we think that making woman feel substantially more comfortable is worth ...
Manufactured by:LGP Consulting, Inc. based inWood River, ILLINOIS (USA)
The PLUGGO™ decapper is an automated system for the safe removal of original stoppers from vacuum tubes. Carousel based automated bench top decapper. Handles all standard vacuum collection ...
Manufactured by:Bristol Myers Squibb Corporate based inNew York, NEW YORK (USA)
Low blood counts are common with DROXIA, including low red blood cells, white blood cells, and platelets, and can be severe and life-threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and during treatment with DROXIA. Your healthcare provider may ...
Manufactured by:Masimo Corp based inIrvine, CALIFORNIA (USA)
LiDCO Antmed closed sets allow for a safe, simple to use and cost effective way to reduce blood wastage when sampling and reduce risk of ...
Manufactured by:Viscot Medical, LLC based inEast Hanover, NEW JERSEY (USA)
Better joint & spinal procedures with Accu-mark: Single use design - decreases the risk of infection. Accurate - ink & metal marker always aligned assuring a mark in the desired spot. Fast & safe - quickest way to mark, less exposure to ...
Manufactured by:ConvaTec Inc. based inBerkshire, UNITED KINGDOM
A dressing designed in consultation with burn surgeons and nurses AQUACEL® Ag Burn helps to: Minimize dressing changes and related pain1-5. Reduce risk of infection*6-7 due to ionic silver – a proven antimicrobial – that kills a broad spectrum of pathogens8. Customize the dressing to patient needs because it is available in larger rectangular ...
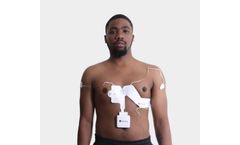
Manufactured by:QT Medical Inc. based inDiamond Bar, CALIFORNIA (USA)
The patented proprietary electrode strip has all 10 individual electrodes pre-positioned and pre-connected. Not only does it enhance efficiency, accuracy and consistency in electrode placement, PCA 500 enables patients to do a medical standard 12-lead ECG in the comfort of their homes. ...
Manufactured by:ITL BioMedical based inVictoria, AUSTRALIA
The automatically closing KV 100 EH Catheter Valve is comfortable used with one hand and thereby provides for a discrete everyday use. It is not possible to connect a urine bag. The automatically closing KV 200 EH Catheter Valve is comfortable used with one hand and thereby provides for a discrete everyday use. A urine bag can be connected by means of the KP 200 Coupling ...