Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (USA)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Influenza B Equipment Supplied In USA
29 equipment items found
Manufactured by:Biosynth Ltd based inCompton, UNITED KINGDOM
SHELF LIFE: 5 years from date of manufacture, SOURCE: Influenza B virus, strain B/Jiangsu/10/03, CULTURE SYSTEM: Embryonated eggs, PRESENTATION: Presented as frozen liquid in Allantoic fluid, INACTIVATION: Heat inactivated. Inactivation of the virus is confirmed by inoculation of a fertile egg without losing vigour in the embryo. ...
Manufactured by:Lumiquick Diagnostics, Inc based inSanta Clara, CALIFORNIA (USA)
Influenza A & B Test is an in vitro immunochromatographic assay for the qualitative detection of influenza type A and type B nucleoprotein antigens in nasopharyngeal swab, nasal swab and nasal aspirate samples. The identification is based on the monoclonal antibodies specific for the nucleoprotein of ...
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
BUDDI™ is an image analyzer that evaluates the line signal intensities on the test ...
Manufactured by:OPTI Medical Systems, Inc. based inRoswell, GEORGIA (US) (USA)
OPTI Medical Systems has obtained CE marking for its OPTI SARS-CoV-2/Influenza A/B RT-PCR Test for identification and differentiation of SARS-CoV-2, influenza A, and influenza B RNA. The test is available in countries accepting the CE mark. This respiratory panel distinguishes between SARS-CoV-2 and ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
Intended use: Hardy Diagnostics Viral Transport Medium is recommended for the collection and transport of clinical specimens for the recovery of viral agents including, but not limited to, Herpes Simplex Type I, Herpes Simplex Type II, Cytomegalovirus (CMV), Influenza A, Influenza B, Respiratory Syncytial Virus (RSV), Echovirus, Adenovirus, etc. ...
Manufactured by:Co-Diagnostics, Inc. based inSalt Lake City, UTAH (USA)
The Logix Smart™ ABC (Influenza A/B, SARS-CoV-2) Test kit is an in vitro diagnostic test that uses our patented CoPrimer™ technology for the qualitative detection of the RNA from the Influenza A, Influenza B, and SARS-CoV-2 viruses. The multiplex test operates using a single step real-time reverse ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
Status Flu A & B is a CLIA Waived in vitro rapid qualitative test that detects Influenza Types A and B antigens directly from nasal or nasopharyngeal swab ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
Influenza is a highly contagious viral infection of the nose, throat, and lungs that occurs most often in the late fall, winter, and early spring. Since other viruses infecting respiratory track have similar symptoms, it is critical to properly diagnose influenza viral infection before any treatment. Flu A&B Plus differentiates ...
Manufactured by:Pathnostics based inIrvine, CALIFORNIA (USA)
The Pathnostics Respiratory Pathogens Test is a highly specific and sensitive multiplex PCR for the nucleic acid detection of SARS-Cov-2, Influenza A, Influenza B, or respiratory syncytial virus (RSV). Using one nasal swab, our Respiratory Pathogens Test will help providers diagnose patients and give appropriate therapy ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
The only FDA EUA visual read rapid test for simultaneous detection and differential diagnosis of SARS-CoV-2, Influenza Type A & Influenza Type B Antigen in nasopharyngeal swab ...
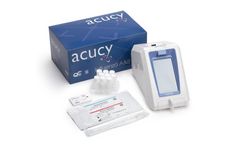
Manufactured by:Sekisui Diagnostics, LLC based inBurlington, MASSACHUSETTS (USA)
With the Acucy® Influenza A&B Test, clinicians can test and provide fast and accurate results that aid in the treatment of a patient in one office visit, enhancing the patient experience, reducing the spread of infection and improving patient ...
Distributed by:Alban Scientific, Inc. based inSt. Louis, MISSOURI (USA)
The BD Veritor™ Plus System offers convenient point-of-care testing for SARS-CoV-2*, Influenza A+B, Group A Streptococcus, and respiratory syncytial virus and combines rapid, reliable results with ease of implementation and simple workflow ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Oseltamivir phosphate (GS 4104) is a neuraminidase inhibitor recommended for the treatment and prophylaxis of influenza A and ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
Respiratory diseases caused by SARS-CoV-2, RSV, and Influenza virus have similar first clinical symptoms, and accurate identification is crucial for better patient care. Gold Standard Diagnostics’ new multiplex assays have been specifically designed to detect and differentiate these viral infections in the same PCR run. The Mplex SARS-CoV-2+, Flu A, Flu B ...
by:ni2o, Inc. based inProvidence, RHODE ISLAND (USA)
Countering Communicable Diseases with our Five-Minute Test. Worldwide Testing Challenges: COVID-19 has opened our eyes to the many communities who don’t have adequate disease detection technology. Ni2o’s RapidTest instrument will allow for a variety of diseases to be detected from samples collected via a non-invasive method, removing the need for specialized testing equipment and ...
Manufactured by:ProImmune Ltd. based inOxford, UNITED KINGDOM
ProM2® MHC Class II Monomers, biotinylated enabling specialist assays for defined MHC Class II peptide target interactions. Best in class MHC Class II Monomers: ProM2® Class II MHC Monomers are pre-biotinylated and can be readily adapted either to detect and separate single-antigen specific T cells or implement specialist solid or solution phase immunoassays for measuring interactions of ...
Manufactured by:Bio Fire Diagnostics based inSalt Lake City, UTAH (USA)
Pneumonia patients are frequently over-treated with antibiotics because it is difficult to quickly identify the pathogen responsible for their symptoms. The BioFire Pneumonia Panel delivers fast, accurate pathogen identification in about one hour, which may allow physicians to put patients on appropriate therapy ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
A rapid immunoassay for the simultaneous direct detection and differential diagnosis of SARS-CoV-2, Influenza Type A and Type B antigen from anterior nasal (AN) or nasopharyngeal (NP) swab specimens. Kit includes extraction buffer vials and flocked nasopharyngeal (NP) swabs for superior specimen collection and patient comfort, and provides a visual read-out in 15 ...
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
The test is offered in BioGX’s trusted, easy to use Sample-Ready™ format where all required reagents for real-time PCR are lyophilized in a single tube. BioGX provided tube snaps into a test-specific position on the BD MAX™ total nucleic acid extraction cartridge enabling Sample-to-Answer molecular testing. BioGX kits can be shipped anywhere in the world with no refrigeration ...
by:Aphios Corporation based inWoburn, MASSACHUSETTS (USA)
Flu – A Serious, Deadly Disease with Pandemic Potential. In 1918, the Spanish flu (H1N1 influenza virus) rapidly killed 50 to 100 million people worldwide, affecting one of every five Americans. Today, influenza remains a common and serious respiratory illness that contributes to the reduction of quality of life and results in a significant loss of manpower hours each year. Every year, 40 ...