Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Injectable Long Equipment Supplied In Usa
7 equipment items found
Manufactured by:Virpax Pharmaceuticals based inBerwyn, PENNSYLVANIA (USA)
Ultra-Long-Acting Local Anesthetics: Liposomal Encapsulated Bupivacaine; Sustained Non-Opioid Pain Management in Prolonged Field Care and Hospital ...
Manufactured by:Windgap Medical, Inc. based inWatertown, MASSACHUSETTS (USA)
Current long-acting injectables require complicated preparation steps, often with dosing and degradation issues. Our in-device mixing mechanisms automate and control this process to protect the medication, the patient, and the ...
Manufactured by:Alkermes based inDublin 4, IRELAND
It is an effective atypical antipsychotic that is well tolerated, based on extensive clinical experience including long-term use. The authorised formulation of Risperdal Consta is a prolonged-release formulation for intramuscular (i.m.) injection containing 12.5, 25, 37.5, or 50 mg ...
Manufactured by:Pion based inBillerica, MASSACHUSETTS (USA)
The Scissor N3 is an advanced in vitro model designed to simulate the subcutaneous injection of biopharmaceuticals. This device is particularly useful during the formulation development phase, enabling researchers to perform rank ordering of formulations and excipient screening. It allows for a thorough understanding of how a drug behaves post subcutaneous injection and its subsequent release, ...
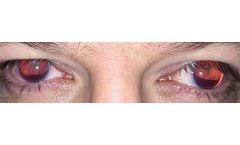
Manufactured by:VEO Ophthalmics, LLC based inCincinnati, OHIO (USA)
Managing congenital aniridia and acquired iris defects — including, but not limited to, traumatic iris defects and traumatic mydriasis — is often challenging. When iris reconstruction is necessary, the CUSTOMFLEX® ARTIFICIALIRIS is a unique device that offers both surgeons and patients important ...
Manufactured by:Clearside Biomedical based inAlpharetta, GEORGIA (US) (USA)
CLS-AX (axitinib injectable suspension) is a proprietary suspension of axitinib for suprachoroidal use. Axitinib is a tyrosine kinase inhibitor (TKI) currently approved to treat renal cell cancer that achieves pan-VEGF blockade, directly inhibiting VEGF receptors-1, -2, and -3 with high potency and specificity. We believe this broad VEGF blockade may have efficacy advantages over ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
FHD-609 is a PROTAC degrader and inhibitor of BRD9 (Bromodomain-containing protein 9). FHD-609 targets to ncBAF, can be used for research of wide range of cancers that contain a mutation in a BAF complex subunit. FHD-609 in combination with Telomelysin or INO5401, may play a role in adrenocortical carcinoma (ACC) treatment. (Blue: BRD9 ligand-6 (HY-49393), Black: linker (HY-168309); Pink: ...