Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Interventional Fluoroscopy Procedures Equipment Supplied In Usa
36 equipment items found
by:Clear Guide Medical based inBaltimore, MARYLAND (USA)
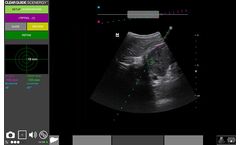
Seamless AR targeting: Perform interventional procedures with clear guidance to just the right spot, CT/MRI clarity + Ultrasound ...
Manufactured by:SentreHEART, Inc. based inPleasanton, CALIFORNIA (USA)
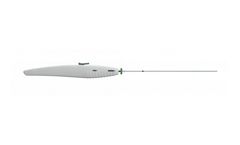
The FindrWIRZ Guide Wire System provides an innovative approach to diagnostic and interventional procedures where control of placement and delivery is ...
Manufactured by:Infraredx, Inc. based inBedford, MASSACHUSETTS (USA)
The GM-30 Inflation Device: The Power of Comfort is in your ...
Manufactured by:Galt Medical Corp. based inGarland, TEXAS (USA)
Galt Medical’s Micro-Access Hemostasis Valve Introducer Kits provide the high quality expected for use during Cardiac Cath Lab and interventional procedures. They are specially designed to provide consistent performance every time. Kink resistant material. Smooth transition between dilator and sheath. .035" and .038" configurations are ...
Manufactured by:Excel Medical Products, Inc. based inWixom, MICHIGAN (USA)
Highlights: Y-Connector configuration with touhy-borst style adapter and rotating male luer Designed to minimize fluid loss during interventional and diagnostic procedures Unique flow adjustment design with large cap for smooth precision control Snap-on cap to prevent dislocation and dropping from the sterile field Increased sidearm angle for ease of use. Details: Available Sizes 4.5 Fr 6 Fr 7 Fr ...
Manufactured by:Precision Coating Company, Inc based inHudson, MASSACHUSETTS (USA)
Typical devices coated in interventional cardiology procedures are guide wires and core wires. PTFE provides low friction to a wire allowing for ease of insertion of a catheter and high release (non-stick) to prevent blood from clotting on the wire. Wire sizes range from 0.013” to 0.022” diameter with coating tolerances from 0.0002” to ...
Manufactured by:Gyn Surgical Solutions based inMarlborough, MASSACHUSETTS (USA)
The Acessa procedure is a minimally invasive, outpatient treatment that involves two small abdominal incisions. It uses controlled radiofrequency energy, heat, to cause coagulative necrosis of the fibroid tissue. The treated tissue softens and shrinks over time, allowing fibroid symptoms to resolve with no suturing of the uterine tissue. Unlike many alternative interventions, the Acessa procedure ...
Manufactured by:EDM Medical Solutions based inNorth Miami, FLORIDA (USA)
The telescopic ultrasound cover allows for a quick and seamless application of the cover over the ultrasound transducer. Telescopic folding suits both 1-person and 2-person setup protocols, and will help you save time before and after each ...
Manufactured by:Transluminal Technologies, LLC based inSyracuse, NEW YORK (USA)
A first-of-its-kind magnesium alloy-based vascular closure device that offers a unique method of quickly achieving stable mechanical vascular closure following percutaneous catheterization for diagnostic or interventional procedures using 5-8 Fr procedural sheaths. The implant consists of a plug and footplate system that when presented to the arteriotomy, effects immediate, stable and reliable ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
The EXOSEAL Vascular Closure Device is designed for a safe, simple, and secure close. The EXOSEAL VCD is indicated for femoral artery puncture site closure, reducing times to hemostasis and ambulation in patients who have undergone diagnostic or interventional catheterization procedures using a standard 5F, 6F, or 7F vascular sheath introducer with up to a 12-cm working ...
Manufactured by:CIVCO Medical Solutions based inCoralville, IOWA (USA)
Envision ultrasound probe covers and scanning pads are activated with a sterile liquid and require no gel. Envision ultrasound viral barriers benefit clinicians and patients during interventions and procedures utilizing ultrasound, such ...
Manufactured by:Philips Healthcare based inAmsterdam, NETHERLANDS
Big Bore RT is designed as a CT simulator to enhance clinical confidence, accelerate time to treat and maximize value of its investment without compromising on patient experience – four dimensions that are essential towards excellent ...
Manufactured by:Image Diagnostics, Inc. (IDI) based inFitchburg, MASSACHUSETTS (USA)
The Aspect ISR G3 is the flagship of the Aspect™ Series table line; designed to be the preferred imaging solution during interventional and vascular procedures. Featuring true iso-centric lateral roll – anatomy on the iso axis remains centered in the fluoroscopic field of ...
Manufactured by:Kollsut based inNorth Miami Beach, FLORIDA (USA)
BIOLIFT™ facelifting threads allows lifting of face skin without surgical intervention. Minimal invasive procedure lasts just 20-25 minutes, casing no damage to the skin and practically no side effects. Immediate effect and short recovery period allows patients to achieve their desired goal easily with no complications. The innovative ultrasonic manufacturing technology gives uniquely ...
by:Supira Medical based inLos Gatos, CALIFORNIA (USA)
Developing a low-profile, high-flow percutaneous ventricular assist device (pVAD) for high risk coronary intervention and cardiogenic ...
Manufactured by:Precision Coating Company, Inc based inHudson, MASSACHUSETTS (USA)
Precision Coating applies medical grade PTFE to devices used in interventional cardiology procedures focused on catheter-based treatment of structural heart diseases. Catheterization involves the insertion of a sheath into a peripheral artery or vein and cannulating the heart under X-ray visualization. A typical procedure would involve the deployment of stents and balloons to care for an acute ...
Manufactured by:Image Diagnostics, Inc. (IDI) based inFitchburg, MASSACHUSETTS (USA)
The 100-4T is designed to be the preferred imaging solution during interventional and vascular procedures. The 100-4T provides longitudinal tabletop float of 32 inches (81.2 cm) in a very compact platform enabling comprehensive coverage throughout the anatomical imaging area without C-arm movement – and does so in a table with an ideal overall length of only 84 ...
Manufactured by:Garwood Medical Devices, LLC based inBuffalo, NEW YORK (USA)
Currently under investigation to study the elimination of biofilm infections on prosthetic knee implants during early intervention procedures while maintaining the current standard of ...
Manufactured by:CIVCO Medical Solutions based inCoralville, IOWA (USA)
NeoGuard is a rolled cover, made with material specially designed for ultrasound use. Recommended for patients and professionals with latex protein sensitivities, these high-quality covers are available for popular endocavity transducers. Covers include colored elastic bands. Sterile gel packets available separately. Not made with natural rubber ...
Manufactured by:CIVCO Medical Solutions based inCoralville, IOWA (USA)
Introducing the Intuit line of transducer covers utilizing CIVCO's premium CIV-Flex™ material and delivering simplified application mechanisms for an improved user experience across all sterile ultrasound procedures. The Intuit series allows convenient placement of gel and simple application onto the transducer along with a three-dimensional “box end” for distortion-free ...