Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Orthopaedic Spine Equipment Supplied In Usa
16 equipment items found
Manufactured by:ECA Medical based inThousand Oaks, CALIFORNIA (USA)
Model 219 Axial: Torque set points from 5 - 20 lb. in. (0.55 - ...
Manufactured by:ECA Medical based inThousand Oaks, CALIFORNIA (USA)
Model 220 T-Handle: Torque set points from 10 – 40 lb. in. (1.12 – ...
Manufactured by:Schaerer Medical AG based inMünsingen, SWITZERLAND
The ortho-trauma and spine table with one electro-hydraulically driven table top movement. Specially for orthopaedic, spine and trauma accessories (Extension devices OTZ, MTS, MIS, Carbonplate and Carbon Spine Frame). AXIS 700 is a AXIS 500 but without seat plate. ...
Manufactured by:Schaerer Medical AG based inMünsingen, SWITZERLAND
The ortho-trauma and spine table with one electro- hydraulically driven table top movement. Specially for orthopaedic, spine and trauma accessories (Ex- tension devices, OTZ, MTS, Carbonplate and Car- bon Spine Frame). Arcus 701 is a Arcus 501 but without seat plate. ...
Manufactured by:Shukla Medical based inSt. Petersburg, FLORIDA (USA)
A universal removal set for all bone screws commonly used in Spine and Orthopedic ...
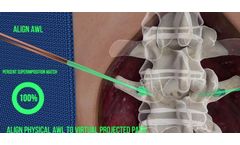
by:OnPoint Surgical, Inc. based inLexington, MASSACHUSETTS (USA)
OnPoint’s Intellectual Property includes a broad range of granted patents and patent applications for Augmented Reality guidance of spinal, neurosurgical, and other surgical procedures. ...
Manufactured by:Viscot Medical, LLC based inEast Hanover, NEW JERSEY (USA)
Mini Red XL Prep Resistant Ink Markers are you unique compared to our standard XL Minis. Red ink is visible on all skin tones ensuring maximum visibility for all patients. Red ink is Gentian Violet Free in compliance with California Proposition 65. All Red XL feature a Regular ...
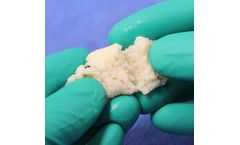
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
OsteGro V Osteo Viable Matrix is the Viable Bone Matrix (VBM) product in the OsteGro family. It is comprised of cancellous bone particles with preserved cells combined with demineralized cortical particles and fibers all processed via Aziyo’s proprietary gentle VBM processes. OsteGro Allograft Bone Matrix, the second product in this family, is a sterile bone matrix comprised of cancellous ...
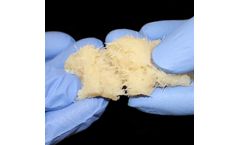
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
ViBone is comprised of cancellous bone particles with preserved cells and demineralized cortical bone particles produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
Viable Bone Matrix for Spine and Orthopedic Procedures. Fiber Viable Bone Matrix: Fiber Viable Bone Matrix (VBM) is made of cancellous bone particles with preserved cells combined with demineralized cortical fiber produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Hensler Surgical Products based inWilmington, NORTH CAROLINA (USA)
ConCelltrate 100 is an osteoinductive bone matrix. ConCelltrate 100 products are verified for osteoinductivity post-sterilization prior to release for distribution. In-vivo test results demonstrate all 5 bone forming elements present (chondrocytes, osteocytes, bone marrow cells, cartilage, and new bone) in our implants. ConCelltrate® 100 does not contain any extrinsic carriers and is made ...
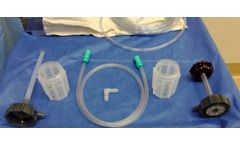
Manufactured by:Hensler Surgical Products based inWilmington, NORTH CAROLINA (USA)
The Hensler Bone Press (HBP) is an advanced surgical tool designed to optimize the collection and separation of autologous bone during neurosurgical and orthopedic spine procedures. Its patented technology efficiently separates bone from fluid, providing a semi-dry, graft-ready material for fusion applications. Engineered for consistent and equal pressure application, the device features a ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-25-Osteo for spinal fusion is a proprietary Phase 3-ready product candidate. All doses of MPC-25-Osteo for the treatment of spinal fusion consist of 25 million MPCs delivered on a collagen ceramic carrier material into the disc space with stabilizing ...
Manufactured by:Spectrum Plastics Group, A DuPont Business based inAlpharetta, GEORGIA (US) (USA)
Polyetheretherketone (PEEK) is a high-strength, high-temperature thermoplastic with superior mechanical and chemical properties that are ideal for medical tubing and other demanding ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-06-ID is a Phase 3 product candidate for the treatment of chronic low back pain caused by disc degeneration (CLBP). It is being developed for patients who have exhausted conservative treatment options, may have failed epidural steroid injections and have no further treatment option other than invasive and costly surgical ...
Manufactured by:Norman Noble, Inc. based inHighland Hts., OHIO (USA)
Proprietary laser machining technologies are custom built by Norman ...