Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Platelet Riched Equipment Supplied In Usa
15 equipment items found
Manufactured by:ProGen | Crown Laboratories, Inc. based inJohnson City, TENNESSEE (USA)
The ProGen Platelet Rich Plasma System delivers the results your practice is looking for with the comfort of knowing that it is manufactured right here in the United States of America. Discover why more physicians are switching to this proven system. ...
Manufactured by:GRAFIX PL, by Smith+Nephew, Inc. based inColumbia, MARYLAND (USA)
The CENTRIO Platelet-Rich Plasma (PRP) System is an advanced point-of-care solution designed to expedite the creation of a biodynamic hematogel from a minimal blood sample of the patient. This system is optimized for treating a variety of wounds, offering coverage for areas up to 2,000 square centimeters. The kit comes fully equipped with wound dressings, ...
Manufactured by:CytoVive, LLC based inTarpon Springs, FLORIDA (USA)
CytoVive specializes in providing advanced FDA-approved Class 2 medical devices under the brands Tropocells and Cellenis, designed for the efficient preparation of autologous Platelet-Rich Plasma (PRP) at the point of care. These PRP kits, intended for single use, leverage proprietary thixotropic gel separator technology. This innovative technology facilitates ...
by:VetStem Biopharma based inPoway, CALIFORNIA (USA)
The principle is to accelerate lagging internal healing processes by amplifying the biological signals that would naturally occur when platelets aggregate at a site of injury. By concentrating platelets and releasing more of those growth factors than would naturally occur – the intended effect is to attract stem cells and stimulate local tissue repair ...
Manufactured by:Celling Biosciences, Inc. based inAustin, TEXAS (USA)
The ART Two Step PRP device features a dual-chamber design allowing the user to perform either leukocyte rich or leukocyte poor platelet-rich plasma. The product uses Celling Bioscience’s patented collection window and dial mechanism allowing precision control to achieve a high yield of platelets in a PRP volume that can be ...
Manufactured by:InGeneron, Inc. based inHouston, TEXAS (USA)
It is an automated and preprogrammed processing device for the heating, agitation, and centrifugation of adipose-derived tissue. Up to four samples can be processed at the same time. Both regenerative cells and platelet-rich plasma can be prepared with the Processing Unit. The Processing Unit is intended to be used in a clean environment by a physician, nurse, or ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
It is Osteoinductive, Osteoconductive, and Osteogenic when mixed with saline, blood, Bone Marrow Aspirate (BMA), Platelet Rich Plasma (PRP), or other cellular components. Indicated for homologous use for the treatment of surgically created or traumatic skeletal ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Designed to process a mixture of autologous whole blood and bone marrow aspirate, the BioCUE BBMA Concentration System represents an evolution in this technique. The system includes all the components to ASPIRATE blood and bone marrow, easily PROCESS the disposable system, and produce an autologous PRP output* to HYDRATE the surgeon’s choice of autograft and/or allograft ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
Incite™ Cortical Fibers are a unique and versatile bone grafting option. A proprietary processing method provides thin bone fibers which expose significant osteoconductive surface area and osteoinductve endogenous growth factors. The ultrafine, entangled fibers are highly malleable to provide exceptional handling properties and support a variety of delivery options for maximum operative ...
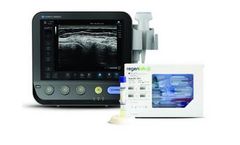
Manufactured by:Konica Minolta Healthcare Americas, Inc. based inWayne, NEW JERSEY (USA)
MSK Ultrasound and Orthobiologics. Leading Technology. Empowering clinical difference. A Partnership Built on Personalized Healthcare. Konica Minolta and RegenLab® USA are innovating the future of MSK and regenerative medicine, together, with a focus on patient ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
ACTIGEN™ Verified Inductive Bone Matrix is a naturally derived, differentiated allograft matrix with robust handling ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Atopaxar (E5555) is a potent, orally active, selective and reversible thrombin receptor protease-activated receptor-1 (PAR-1) antagonist. Atopaxar, an antiplatelet agent, interferes with platelet signaling. Atopaxar can be used for the research of atherothrombotic ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
UNC2881 is an orally active and specific Mer kinase inhibitor, inhibits steady-state Mer kinase phosphorylation with an IC50 value of 22 nM. UNC2881 shows additional inhibition against Axl and Tyro with IC50s of 360 nM and 250 nM, respectively. UNC2881 potently inhibits collagen-induced platelet aggregation, can be used for pathologic thrombosis ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Mofezolac, a non-steroidal anti-inflammatory drug (NSAID), is a selective, reversible and orally active COX-1 inhibitor with an IC50 of 1.44 nM. Mofezolac shows weak inhibitory activity on COX-2 (IC50 of 447 nM). Mofezolac can relieve pain and has anti-inflammatory ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Kartogenin (KGN) is an inducer of chondrogenic tissue formation (EC50: 100 nM). Kartogenin induces chondrogenesis by binding to fibrin A, disrupting its interaction with the transcription factor core binding factor beta subunit (CBFβ), and by modulating the CBFβ-RUNX1 transcriptional program. Kartogenin also promotes tendon-bone junction (TBJ) wound healing by stimulating collagen synthesis. ...